Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors
- PMID: 20072125
- PMCID: PMC2901986
- DOI: 10.1038/nature08675
Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors
Abstract
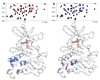
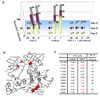
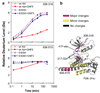
In an effort to find new pharmacological modalities to overcome resistance to ATP-binding-site inhibitors of Bcr-Abl, we recently reported the discovery of GNF-2, a selective allosteric Bcr-Abl inhibitor. Here, using solution NMR, X-ray crystallography, mutagenesis and hydrogen exchange mass spectrometry, we show that GNF-2 binds to the myristate-binding site of Abl, leading to changes in the structural dynamics of the ATP-binding site. GNF-5, an analogue of GNF-2 with improved pharmacokinetic properties, when used in combination with the ATP-competitive inhibitors imatinib or nilotinib, suppressed the emergence of resistance mutations in vitro, displayed additive inhibitory activity in biochemical and cellular assays against T315I mutant human Bcr-Abl and displayed in vivo efficacy against this recalcitrant mutant in a murine bone-marrow transplantation model. These results show that therapeutically relevant inhibition of Bcr-Abl activity can be achieved with inhibitors that bind to the myristate-binding site and that combining allosteric and ATP-competitive inhibitors can overcome resistance to either agent alone.
Figures
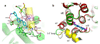

References
-
- Weisberg E, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129. - PubMed
-
- Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6(10):834. - PubMed
-
- Shah NP, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399. - PubMed
-
- Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876. - PubMed
-
- Nagar B, et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62(15):4236. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

