Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils
- PMID: 20072612
- PMCID: PMC2798753
- DOI: 10.1371/journal.ppat.1000715
Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils
Abstract
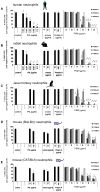
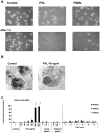
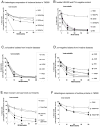
The role of the pore-forming Staphylococcus aureus toxin Panton-Valentine leukocidin (PVL) in severe necrotizing diseases is debated due to conflicting data from epidemiological studies of community-associated methicillin-resistant S. aureus (CA-MRSA) infections and various murine disease-models. In this study, we used neutrophils isolated from different species to evaluate the cytotoxic effect of PVL in comparison to other staphylococcal cytolytic components. Furthermore, to study the impact of PVL we expressed it heterologously in a non-virulent staphylococcal species and examined pvl-positive and pvl-negative clinical isolates as well as the strain USA300 and its pvl-negative mutant. We demonstrate that PVL induces rapid activation and cell death in human and rabbit neutrophils, but not in murine or simian cells. By contrast, the phenol-soluble modulins (PSMs), a newly identified group of cytolytic staphylococcal components, lack species-specificity. In general, after phagocytosis of bacteria different pvl-positive and pvl-negative staphylococcal strains, expressing a variety of other virulence factors (such as surface proteins), induced cell death in neutrophils, which is most likely associated with the physiological clearing function of these cells. However, the release of PVL by staphylococcal strains caused rapid and premature cell death, which is different from the physiological (and programmed) cell death of neutrophils following phagocytosis and degradation of virulent bacteria. Taken together, our results question the value of infection-models in mice and non-human primates to elucidate the impact of PVL. Our data clearly demonstrate that PVL acts differentially on neutrophils of various species and suggests that PVL has an important cytotoxic role in human neutrophils, which has major implications for the pathogenesis of CA-MRSA infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. - PubMed
-
- King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–317. - PubMed
-
- Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. - PubMed
-
- Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S378–S385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

