Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility
- PMID: 20072627
- PMCID: PMC2800191
- DOI: 10.1371/journal.pone.0008682
Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility
Abstract
Background: Most medicinal plants contain a mixture of bioactive compounds, including chemicals that interact with intracellular targets and others that can act as adjuvants to facilitate absorption of polar agents across cellular membranes. However, little is known about synergistic effects between such potential drug candidates and adjuvants. To probe for such effects, we tested the green tea compound epigallocatechin gallate (EGCG) and the membrane permeabilising digitonin on Plasmodium sporozoite motility and viability.
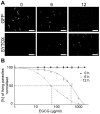
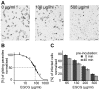
Methodology/principal findings: Green fluorescent P. berghei sporozoites were imaged using a recently developed visual screening methodology. Motility and viability parameters were automatically analyzed and IC50 values were calculated, and the synergism of drug and adjuvant was assessed by the fractional inhibitory concentration index. Validating our visual screening procedure, we showed that sporozoite motility and liver cell infection is inhibited by EGCG at nontoxic concentrations. Digitonin synergistically increases the cytotoxicity of EGCG on sporozoite survival, but shows an additive effect on sporozoite motility.
Conclusions/significance: We proved the feasibility of performing highly reliable visual screens for compounds against Plasmodium sporozoites. We thereby could show an advantage of administering mixtures of plant metabolites on inhibition of cell motility and survival. Although the effective concentration of both drugs is too high for use in malaria prophylaxis, the demonstration of a synergistic effect between two plant compounds could lead to new avenues in drug discovery.
Conflict of interest statement
Figures

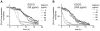


Similar articles
-
Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin.Phytomedicine. 2018 Nov 15;50:213-222. doi: 10.1016/j.phymed.2018.09.169. Epub 2018 Sep 17. Phytomedicine. 2018. PMID: 30466981
-
Screening for potential prophylactics targeting sporozoite motility through the skin.Malar J. 2018 Aug 31;17(1):319. doi: 10.1186/s12936-018-2469-0. Malar J. 2018. PMID: 30170589 Free PMC article.
-
Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates.J Mycol Med. 2019 Jun;29(2):158-167. doi: 10.1016/j.mycmed.2019.01.011. Epub 2019 Feb 20. J Mycol Med. 2019. PMID: 30797684
-
Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy.Clin Nutr. 2013 Dec;32(6):894-903. doi: 10.1016/j.clnu.2013.03.008. Epub 2013 Mar 15. Clin Nutr. 2013. PMID: 23582951 Review.
-
Inhibitory Effects of Eight Green Tea Catechins on Cytochrome P450 1A2, 2C9, 2D6, and 3A4 Activities.J Pharm Pharm Sci. 2016 Apr-Jun;19(2):188-97. doi: 10.18433/J3MS5C. J Pharm Pharm Sci. 2016. PMID: 27518169 Review.
Cited by
-
Modes of Action of Herbal Medicines and Plant Secondary Metabolites.Medicines (Basel). 2015 Sep 8;2(3):251-286. doi: 10.3390/medicines2030251. Medicines (Basel). 2015. PMID: 28930211 Free PMC article. Review.
-
Developing transmission-blocking strategies for malaria control.PLoS Pathog. 2017 Jul 6;13(7):e1006336. doi: 10.1371/journal.ppat.1006336. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28683121 Free PMC article. Review. No abstract available.
-
Combinations of alkaloids affecting different molecular targets with the saponin digitonin can synergistically enhance trypanocidal activity against Trypanosoma brucei brucei.Antimicrob Agents Chemother. 2015 Nov;59(11):7011-7. doi: 10.1128/AAC.01315-15. Epub 2015 Sep 8. Antimicrob Agents Chemother. 2015. PMID: 26349826 Free PMC article.
-
Active migration and passive transport of malaria parasites.Trends Parasitol. 2015 Aug;31(8):357-62. doi: 10.1016/j.pt.2015.04.010. Epub 2015 May 20. Trends Parasitol. 2015. PMID: 26001482 Free PMC article. Review.
-
In Vitro Growth- and Encystation-Inhibitory Efficacies of Matcha Green Tea and Epigallocatechin Gallate Against Acanthameoba Castellanii.Pathogens. 2020 Sep 17;9(9):763. doi: 10.3390/pathogens9090763. Pathogens. 2020. PMID: 32957663 Free PMC article.
References
-
- Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr Drug Med. 2008;9:996–1009. - PubMed
-
- Wink M. Kayser O, Quax W, editors. Bioprospecting: The search for bioactive lead structures from nature. 2007. pp. 97–116. Medicinal Plant Biotechnology. Wiley-VCH.
-
- Schäfer H, Wink M. Medicinally important secondary metabolites in recombinant microorganisms or plants: Progress in alkaloid biosynthesis. Biotechnol J. 2009;4:1684–1703. - PubMed
-
- Harborne JB. Role of secondary metabolites in chemical defence mechanisms in plants. Ciba Found Symp. 1990;154:126–134. - PubMed
-
- Waterman PG. Roles for secondary metabolites in plants. Ciba Found Symp. 1992;171:255–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

