Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice
- PMID: 20072658
- PMCID: PMC2805888
- DOI: 10.1593/neo.91718
Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice
Abstract
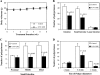
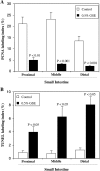
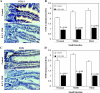
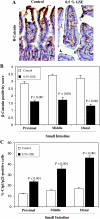
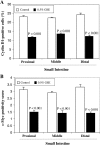
Chemopreventive effects and associated mechanisms of grape seed extract (GSE) against intestinal/colon cancer development are largely unknown. Herein, we investigated GSE efficacy against intestinal tumorigenesis in APC(min/+) mice. Female APC(min/+) mice were fed control or 0.5% GSE (wt/wt) mixed AIN-76A diet for 6 weeks. At the end of the experiment, GSE feeding decreased the total number of intestinal polyps by 40%. The decrease in polyp formation in the small intestine was 42%, which was mostly in its middle (51%) and distal (49%) portions compared with the proximal one. GSE also decreased polyp growth where the number of polyps of 1 to 2 mm in size decreased by 42% and greater than 2 mm in size by 71%, without any significant change in polyps less than 1 mm in size. Immunohistochemical analyses of small intestinal tissue samples revealed a decrease (80%-86%) in cell proliferation and an increase (four- to eight-fold) in apoptosis. GSE feeding also showed decreased protein levels of cyclooxygenase-2 (COX-2) (56%-64%), inducible nitric oxide synthase (iNOS) (58%-60%), and beta-catenin (43%-59%) but an increased Cip1/p21-positive cells (1.9- to 2.6-fold). GSE also decreased cyclin D1 and c-Myc protein levels in small intestine. Together, these findings show the chemopreventive potential of GSE against intestinal polyp formation and growth in APC(min/+) mice, which was accompanied with reduced cell proliferation and increased apoptosis together with down-regulation in COX-2, iNOS, beta-catenin, cyclin D1, and c-Myc expression, but increased Cip1/p21. In conclusion, the present study suggests potential usefulness of GSE for the chemoprevention of human intestinal/colorectal cancer.
Figures
References
-
- Sesink AL, Termont DS, Kleibeuker JH, Van Der Meer R. Red meat and colon cancer: dietary haem, but not fat, has cytotoxic and hyperproliferative effects on rat colonic epithelium. Carcinogenesis. 2000;21:1909–1915. - PubMed
-
- Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–2664. - PubMed
-
- Martínez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. - PubMed
-
- Durak I, Cetin R, Devrim E, Ergüder IB. Effects of black grape extract on activities of DNA turn-over enzymes in cancerous and non cancerous human colon tissues. Life Sci. 2005;76:2995–3000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
