Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction
- PMID: 20072666
- PMCID: PMC2805256
- DOI: 10.1146/annurev.fluid.010908.165136
Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction
Abstract
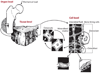
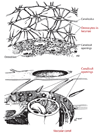
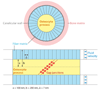
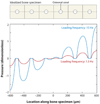
Much recent evidence suggests that bone cells sense their mechanical environment via interstitial fluid flow. In this review, we summarize theoretical and experimental approaches to quantify fluid and solute transport in bone, starting with the early investigations of fluid shear stress applied to bone cells. The pathways of bone interstitial fluid and solute movement are high-lighted based on recent theoretical models, as well as a new generation of tracer experiments that have clarified and refined the structure and function of the osteocyte pericellular matrix. Then we trace how the fluid-flow models for mechanotransduction have evolved as new ultrastructural features of the osteocyte lacunar-canalicular porosity have been identified and how more recent in vitro fluid-flow and cell-stretch experiments have helped elucidate at the molecular level the possible pathways for cellular excitation in bone.
Figures
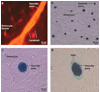
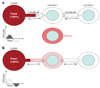

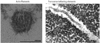

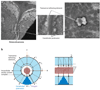
References
-
- Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am. J. Physiol. 1999;276:E171–E178. - PubMed
-
- Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes—a cytoskeleton-dependent process. Biochem. Biophys. Res. Commun. 1996;225:62–68. - PubMed
-
- Anderson EJ, Kaliyamoorthy S, Iwan J, Alexander D, Knothe Tate ML. Nano-microscale models of periosteocytic flow show differences in stresses imparted to cell body and processes. Ann. Biomed. Eng. 2005;33:52–62. - PubMed
-
- Ayasaka N, Kondo T, Goto T, Kido MA, Nagata E, Tanaka T. Differences in the transport systems between cementocytes and osteocytes in rats using microperoxidase as a tracer. Arch. Oral Biol. 1992;37:363–369. - PubMed
-
- Bakker A, Klein-Nulend J, Burger E. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem. Biophys. Res. Commun. 2004;320:1163–1168. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
