Mapping of oxidative stress response elements of the caveolin-1 promoter
- PMID: 20072934
- PMCID: PMC3707498
- DOI: 10.1007/978-1-60761-411-1_29
Mapping of oxidative stress response elements of the caveolin-1 promoter
Abstract
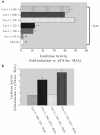
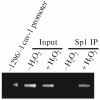
According to the "free radical theory" of aging, normal aging occurs as the result of tissue damages inflicted by reactive oxygen species (ROS). ROS are known to induce cellular senescence, and senescent cells are believed to contribute to organismal aging. The molecular mechanisms that mediate the cellular response to oxidants remain to be fully identified. We have shown that oxidative stress induces cellular senescence through activation of the caveolin-1 promoter and upregulation of caveolin-1 protein expression. Here, we describe how reactive oxygen species activate the caveolin-1 promoter and how the signaling may be assayed. These approaches provide insight into the functional role of caveolin-1 and potentially allow the identification of novel ROS-regulated genes that are part of the signaling machinery regulating cellular senescence/aging.
Figures
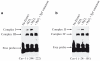
Similar articles
-
Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements.Cancer Res. 2006 Nov 15;66(22):10805-14. doi: 10.1158/0008-5472.CAN-06-1236. Cancer Res. 2006. PMID: 17108117 Free PMC article.
-
Caveolin-1, cellular senescence and age-related diseases.Mech Ageing Dev. 2011 Nov-Dec;132(11-12):533-42. doi: 10.1016/j.mad.2011.11.001. Epub 2011 Nov 12. Mech Ageing Dev. 2011. PMID: 22100852 Free PMC article. Review.
-
Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence.Mol Biol Cell. 2013 Jun;24(12):1852-62. doi: 10.1091/mbc.E12-09-0666. Epub 2013 May 1. Mol Biol Cell. 2013. PMID: 23637463 Free PMC article.
-
Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6).J Biol Chem. 2015 Feb 13;290(7):4202-14. doi: 10.1074/jbc.M114.598268. Epub 2014 Dec 15. J Biol Chem. 2015. PMID: 25512378 Free PMC article.
-
Caveolin-1, a master regulator of cellular senescence.Cancer Metastasis Rev. 2020 Jun;39(2):397-414. doi: 10.1007/s10555-020-09875-w. Cancer Metastasis Rev. 2020. PMID: 32279119 Free PMC article. Review.
Cited by
-
Adenosine 5'-triphosphate-sensitive potassium channel activator induces the up-regulation of caveolin-1 expression in a rat brain tumor model.Cell Mol Neurobiol. 2011 May;31(4):629-34. doi: 10.1007/s10571-011-9658-5. Epub 2011 Feb 18. Cell Mol Neurobiol. 2011. PMID: 21331626 Free PMC article.
-
Neoatherosclerosis after Drug-Eluting Stent Implantation: Roles and Mechanisms.Oxid Med Cell Longev. 2016;2016:5924234. doi: 10.1155/2016/5924234. Epub 2016 Jun 30. Oxid Med Cell Longev. 2016. PMID: 27446509 Free PMC article. Review.
-
Caveolae as Potential Hijackable Gates in Cell Communication.Front Cell Dev Biol. 2020 Oct 27;8:581732. doi: 10.3389/fcell.2020.581732. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195223 Free PMC article. Review.
-
Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma.Mol Vis. 2011;17:2878-88. Epub 2011 Nov 9. Mol Vis. 2011. PMID: 22128235 Free PMC article.
-
[Caveolin-1 in relation with mitochondria and cancer metabolism-a promising target for cancer therapy].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2017 Oct 1;34(5):803-806. doi: 10.7507/1001-5515.201703037. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2017. PMID: 29761970 Free PMC article. Review. Chinese.
References
-
- Chen QM. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann N Y Acad Sci. 2000;908:111–125. - PubMed
-
- Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. - PubMed
-
- Black EJ, Clark W, Gillespie DA. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr Biol. 2000;10:1119–1122. - PubMed
-
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

