Amino acid interaction preferences in proteins
- PMID: 20073083
- PMCID: PMC2866284
- DOI: 10.1002/pro.339
Amino acid interaction preferences in proteins
Abstract
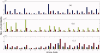
Understanding the key factors that influence the interaction preferences of amino acids in the folding of proteins have remained a challenge. Here we present a knowledge-based approach for determining the effective interactions between amino acids based on amino acid type, their secondary structure, and the contact based environment that they find themselves in the native state structure as measured by their number of neighbors. We find that the optimal information is approximately encoded in a 60 x 60 matrix describing the 20 types of amino acids in three distinct secondary structures (helix, beta strand, and loop). We carry out a clustering scheme to understand the similarity between these interactions and to elucidate a nonredundant set. We demonstrate that the inferred energy parameters can be used for assessing the fit of a given sequence into a putative native state structure.
Figures


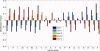
Similar articles
-
Importance of native-state topology for determining the folding rate of two-state proteins.J Chem Inf Comput Sci. 2003 Sep-Oct;43(5):1481-5. doi: 10.1021/ci0340308. J Chem Inf Comput Sci. 2003. PMID: 14502481
-
NIAS-Server: Neighbors Influence of Amino acids and Secondary Structures in Proteins.J Comput Biol. 2017 Mar;24(3):255-265. doi: 10.1089/cmb.2016.0074. Epub 2016 Aug 5. J Comput Biol. 2017. PMID: 27494258
-
Fuzzy cluster analysis of simple physicochemical properties of amino acids for recognizing secondary structure in proteins.Protein Sci. 1995 Jun;4(6):1178-87. doi: 10.1002/pro.5560040616. Protein Sci. 1995. PMID: 7549882 Free PMC article.
-
Amino acid conformational preferences and solvation of polar backbone atoms in peptides and proteins.J Mol Biol. 2000 Jul 28;300(5):1335-59. doi: 10.1006/jmbi.2000.3901. J Mol Biol. 2000. PMID: 10903873
-
Amino acid network for the discrimination of native protein structures from decoys.Curr Protein Pept Sci. 2014;15(6):522-8. doi: 10.2174/1389203715666140724084709. Curr Protein Pept Sci. 2014. PMID: 25059328 Review.
Cited by
-
ATLIGATOR: editing protein interactions with an atlas-based approach.Bioinformatics. 2022 Nov 30;38(23):5199-5205. doi: 10.1093/bioinformatics/btac685. Bioinformatics. 2022. PMID: 36259946 Free PMC article.
-
Hydrophobicity-Based Force Field In Enzymes.ACS Omega. 2024 Feb 7;9(7):8188-8203. doi: 10.1021/acsomega.3c08728. eCollection 2024 Feb 20. ACS Omega. 2024. PMID: 38405467 Free PMC article.
-
Structural motifs in protein cores and at protein-protein interfaces are different.Protein Sci. 2021 Feb;30(2):381-390. doi: 10.1002/pro.3996. Epub 2020 Nov 20. Protein Sci. 2021. PMID: 33166001 Free PMC article.
-
Alveolin proteins in the Toxoplasma inner membrane complex form a highly interconnected structure that maintains parasite shape and replication.PLoS Biol. 2024 Sep 12;22(9):e3002809. doi: 10.1371/journal.pbio.3002809. eCollection 2024 Sep. PLoS Biol. 2024. PMID: 39264987 Free PMC article.
-
Circles within circles: crosstalk between protein Ser/Thr/Tyr-phosphorylation and Met oxidation.BMC Bioinformatics. 2013;14 Suppl 14(Suppl 14):S14. doi: 10.1186/1471-2105-14-S14-S14. Epub 2013 Oct 9. BMC Bioinformatics. 2013. PMID: 24267725 Free PMC article.
References
-
- Chothia C. Proteins. One thousand families for the molecular biologist. Nature. 1992;357:543–544. - PubMed
-
- Banavar JR, Cieplak M, Maritan A. Lattice tube model of proteins. Phys Rev Lett. 2004;93:238101(1–4). - PubMed
-
- Banavar JR, Maritan A. Colloquium: geometrical approach to protein folding: a tube picture. Rev Mod Phys. 2003;75:23–34.
-
- Banavar JR, Maritan A. Physics of proteins. Annu Rev Biophys Biomol Struct. 2007;36:261–280. - PubMed
-
- Banavar JR, Hoang TX, Maritan A, Seno F, Trovato A. Unified perspective on proteins: a physics approach. Phys Rev. 2004;70:041905(1–25). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

