Bispecific antibodies for cancer therapy: the light at the end of the tunnel?
- PMID: 20073127
- PMCID: PMC2791310
- DOI: 10.4161/mabs.1.6.10015
Bispecific antibodies for cancer therapy: the light at the end of the tunnel?
Abstract
With 23 approvals in the US and other countries and four approvals outside US, antibodies are now widely recognized as therapeutic molecules. The therapeutic and commercial successes met by rituximab, trastuzumab, cetuximab and other mAbs have inspired antibody engineers to improve the efficacy of these molecules. Consequently, a new wave of antibodies with engineered Fc leading to much higher effector functions such as antibody-dependent cell-mediated cytotoxicity or complement-dependent cytotoxicity is being evaluated in the clinic, and several approvals are expected soon. In addition, research on a different class of antibody therapeutics, bispecific antibodies, has recently led to outstanding clinical results, and the first approval of the bispecific antibody catumaxomab, a T cell retargeting agent that was approved in the European Union in April 2009. This review describes the most recent advances and clinical study results in the field of bispecific antibodies, a new class of molecules that might outshine conventional mAbs as cancer immunotherapeutics in a near future.
Figures
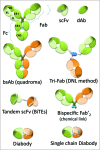
References
-
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. - PubMed
-
- Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–631. - PubMed
-
- Chames P, Baty D. Bispecific antibodies for cancer therapy. Curr Opin Drug Discov Devel. 2009;12:276–283. - PubMed
-
- Saerens D, Ghassabeh GH, Muyldermans S. Single-domain antibodies as building blocks for novel therapeutics. Curr Opin Pharmacol. 2008;8:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
