Parkin-mediated ubiquitin signalling in aggresome formation and autophagy
- PMID: 20074049
- PMCID: PMC2846638
- DOI: 10.1042/BST0380144
Parkin-mediated ubiquitin signalling in aggresome formation and autophagy
Abstract
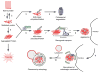
Understanding how cells handle and dispose of misfolded proteins is of paramount importance because protein misfolding and aggregation underlie the pathogenesis of many neurodegenerative disorders, including PD (Parkinson's disease) and Alzheimer's disease. In addition to the ubiquitin-proteasome system, the aggresome-autophagy pathway has emerged as another crucial cellular defence system against toxic build-up of misfolded proteins. In contrast with basal autophagy that mediates non-selective, bulk clearance of misfolded proteins along with normal cellular proteins and organelles, the aggresome-autophagy pathway is increasingly recognized as a specialized type of induced autophagy that mediates selective clearance of misfolded and aggregated proteins under the conditions of proteotoxic stress. Recent evidence implicates PD-linked E3 ligase parkin as a key regulator of the aggresome-autophagy pathway and indicates a signalling role for Lys(63)-linked polyubiquitination in the regulation of aggresome formation and autophagy. The present review summarizes the current knowledge of the aggresome-autophagy pathway, its regulation by parkin-mediated Lys(63)-linked polyubiquitination, and its dysfunction in neurodegenerative diseases.
Figures
References
-
- Gregersen N. Protein misfolding disorders: pathogenesis and intervention. J Inherit Metab Dis. 2006;29:456–470. - PubMed
-
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. - PubMed
-
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

