Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as neurokinin 1 receptor internalization
- PMID: 20074214
- PMCID: PMC2857979
- DOI: 10.1111/j.1460-9568.2009.07075.x
Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as neurokinin 1 receptor internalization
Abstract
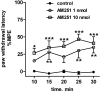
The contribution of CB1 receptors in the spinal cord to cannabinoid analgesia is still unclear. The objective of this study was to investigate the effect of CB1 receptors on substance P release from primary afferent terminals in the spinal cord. Substance P release was measured as neurokinin 1 (NK1) receptor internalization in lamina I neurons. It was induced in spinal cord slices by dorsal root stimulation and in live rats by a noxious stimulus. In spinal cord slices, the CB1 receptor antagonists AM251, AM281 and rimonabant partially but potently inhibited NK1 receptor internalization induced by electrical stimulation of the dorsal root. This was due to an inhibition of substance P release and not of NK1 receptor internalization itself, because AM251 and AM281 did not inhibit NK1 receptor internalization induced by exogenous substance P. The CB1 receptor agonist ACEA increased NK1 receptor internalization evoked by dorsal root stimulation. The effects of AM251 and ACEA cancelled each other. In vivo, AM251 injected intrathecally decreased NK1 receptor internalization in spinal segments L5 and L6 induced by noxious hind paw clamp. Intrathecal AM251 also produced analgesia to radiant heat stimulation of the paw. The inhibition by AM251 of NK1 receptor internalization was reversed by antagonists of mu-opioid and GABA(B) receptors. This indicates that CB1 receptors facilitate substance P release by inhibiting the release of GABA and opioids next to primary afferent terminals, producing disinhibition. This results in a pronociceptive effect of CB1 receptors in the spinal cord.
Figures








Comment in
-
Spinal cannabinoids--a double-edged sword? (Commentary on Zhang et al.).Eur J Neurosci. 2010 Jan;31(2):223-4. doi: 10.1111/j.1460-9568.2010.07125.x. Epub 2010 Jan 13. Eur J Neurosci. 2010. PMID: 20074211 No abstract available.
Similar articles
-
GABA(A) receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord.Neuroscience. 2005;130(4):1013-27. doi: 10.1016/j.neuroscience.2004.10.019. Neuroscience. 2005. PMID: 15652997
-
μ-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain.Neuroscience. 2014 May 16;267:67-82. doi: 10.1016/j.neuroscience.2014.02.023. Epub 2014 Feb 26. Neuroscience. 2014. PMID: 24583035 Free PMC article.
-
Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord.Neuroscience. 2003;118(2):535-45. doi: 10.1016/s0306-4522(02)00977-6. Neuroscience. 2003. PMID: 12699788
-
Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release.J Neurosci. 2005 Apr 6;25(14):3651-60. doi: 10.1523/JNEUROSCI.0252-05.2005. J Neurosci. 2005. PMID: 15814796 Free PMC article.
-
Src family kinases mediate the inhibition of substance P release in the rat spinal cord by μ-opioid receptors and GABA(B) receptors, but not α2 adrenergic receptors.Eur J Neurosci. 2010 Sep;32(6):963-73. doi: 10.1111/j.1460-9568.2010.07335.x. Epub 2010 Aug 19. Eur J Neurosci. 2010. PMID: 20726886 Free PMC article.
Cited by
-
Acetaminophen inhibits diacylglycerol lipase synthesis of 2-arachidonoyl glycerol: Implications for nociception.Cell Rep Med. 2025 Jun 17;6(6):102139. doi: 10.1016/j.xcrm.2025.102139. Epub 2025 May 16. Cell Rep Med. 2025. PMID: 40381619 Free PMC article.
-
Cannabinoid 1 receptor knockout mice display cold allodynia, but enhanced recovery from spared-nerve injury-induced mechanical hypersensitivity.Mol Pain. 2016 May 20;12:1744806916649191. doi: 10.1177/1744806916649191. Print 2016. Mol Pain. 2016. PMID: 27206660 Free PMC article.
-
Endocannabinoid signaling in stress, nausea, and vomiting.Neurogastroenterol Motil. 2025 Mar;37(3):e14911. doi: 10.1111/nmo.14911. Epub 2024 Sep 2. Neurogastroenterol Motil. 2025. PMID: 39223918 Free PMC article. Review.
-
Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons.Neuroscience. 2014 Jan 3;256:178-94. doi: 10.1016/j.neuroscience.2013.10.054. Epub 2013 Oct 31. Neuroscience. 2014. PMID: 24184981 Free PMC article.
-
The interaction between intrathecal administration of low doses of palmitoylethanolamide and AM251 in formalin-induced pain related behavior and spinal cord IL1-β expression in rats.Neurochem Res. 2012 Apr;37(4):778-85. doi: 10.1007/s11064-011-0672-2. Epub 2011 Dec 27. Neurochem Res. 2012. PMID: 22201038
References
-
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. - PMC - PubMed
-
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. - PubMed
-
- Aimone LD, Yaksh TL. Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides. 1989;10:1127–1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

