Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity
- PMID: 20074529
- PMCID: PMC2824926
- DOI: 10.1016/j.cmet.2009.11.008
Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity
Abstract
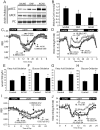
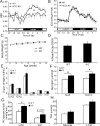
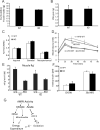
Activation of AMP-activated protein kinase (AMPK) is thought to convey many of the beneficial effects of exercise via its inhibitory effect on acetyl-CoA carboxylase 2 (ACC2) and promotion of fatty acid oxidation. Hence, AMPK and ACC have become major drug targets for weight loss and improved insulin action. However, it remains unclear whether or how activation of the fatty acid oxidation pathway without a concomitant increase in energy expenditure could be beneficial. Here, we have used either pharmacological (administration of the AMPK agonist 5(') aminoimidazole-4-carboxamide-riboside) or genetic means (mutation of the ACC2 gene in mice) to manipulate fatty acid oxidation to determine whether this is sufficient to promote leanness. Both of these strategies increased whole-body fatty acid oxidation without altering energy expenditure or adiposity. We conclude that negative energy balance is a prerequisite for weight reduction, and increased fatty acid oxidation per se has little, if any, effect to reduce adiposity.
2010 Elsevier Inc.
Figures

References
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
-
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–1346. - PubMed
-
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA, Kraegen EW. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. - PMC - PubMed
-
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

