Isoform- and dose-sensitive feedback interactions between paired box 6 gene and delta-catenin in cell differentiation and death
- PMID: 20074565
- PMCID: PMC2885963
- DOI: 10.1016/j.yexcr.2010.01.006
Isoform- and dose-sensitive feedback interactions between paired box 6 gene and delta-catenin in cell differentiation and death
Abstract
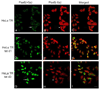
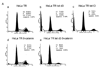
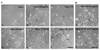
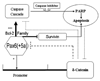
Pax6, a mammalian homolog of the Drosophila paired box gene family member expressed in stem and progenitor cells, resides at the top of the genetic hierarchy in controlling cell fates and morphogenesis. While Pax6 activation can lead to mitotic arrest, premature neurogenesis, and apoptosis, the underlying molecular mechanisms have not been resolved. Here we report that either Pax6(+5a) or Pax6(-5a) was sufficient to promote, whereas their knockdown reduced the expression of delta-catenin (CTNND2), a neural specific member of the armadillo/beta-catenin superfamily. Pax6(+5a) elicited stronger effects on delta-catenin than Pax6(-5a). Inducible Pax6(+5a) expression demonstrated a biphasic and dose-dependent regulation of delta-catenin expression and cell fates. A moderate upregulation of Pax6(+5a) promoted delta-catenin expression and induced neurite-like cellular protrusions, but increasing expression of Pax6(+5a) reversed these processes. Furthermore, sustained high expression of Pax6(+5a) triggered apoptosis as determined by the reduction of phospho-Bad, Bcl-2, survivin and procaspases, as well as the increases in Bax and cleaved poly(ADP-ribose) polymerase. Importantly, re-introducing delta-catenin by ectopic expression elicited a feedback suppression on Pax6(+5a) expression and reduced Pax6(+5a) induced apoptosis. Therefore, delta-catenin expression is not only controlled by Pax6, but it also provides a feedback suppression mechanism for their functional interactions with important implications in cellular morphogenesis, apoptosis, and cancer.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Positive autoregulation of the transcription factor Pax6 in response to increased levels of either of its major isoforms, Pax6 or Pax6(5a), in cultured cells.BMC Dev Biol. 2006 May 25;6:25. doi: 10.1186/1471-213X-6-25. BMC Dev Biol. 2006. PMID: 16725027 Free PMC article.
-
Pax6 regulates the expression of Dkk3 in murine and human cell lines, and altered responses to Wnt signaling are shown in FlpIn-3T3 cells stably expressing either the Pax6 or the Pax6(5a) isoform.PLoS One. 2014 Jul 16;9(7):e102559. doi: 10.1371/journal.pone.0102559. eCollection 2014. PLoS One. 2014. PMID: 25029272 Free PMC article.
-
Dynamic Pax6 expression during the neurogenic cell cycle influences proliferation and cell fate choices of retinal progenitors.Neural Dev. 2009 Aug 17;4:32. doi: 10.1186/1749-8104-4-32. Neural Dev. 2009. PMID: 19686589 Free PMC article.
-
Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb.Dev Biol. 2006 Apr 15;292(2):486-505. doi: 10.1016/j.ydbio.2005.12.041. Epub 2006 Feb 7. Dev Biol. 2006. PMID: 16464444
-
Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in α-cell differentiation, glucagon biosynthesis and secretion.Diabetes Obes Metab. 2011 Oct;13 Suppl 1:31-8. doi: 10.1111/j.1463-1326.2011.01445.x. Diabetes Obes Metab. 2011. PMID: 21824254 Review.
Cited by
-
PAX6 overexpression is associated with the poor prognosis of invasive ductal breast cancer.Oncol Lett. 2015 Sep;10(3):1501-1506. doi: 10.3892/ol.2015.3434. Epub 2015 Jun 29. Oncol Lett. 2015. PMID: 26622698 Free PMC article.
-
A new genome scan for primary nonsyndromic vesicoureteric reflux emphasizes high genetic heterogeneity and shows linkage and association with various genes already implicated in urinary tract development.Mol Genet Genomic Med. 2014 Jan;2(1):7-29. doi: 10.1002/mgg3.22. Epub 2013 Jul 7. Mol Genet Genomic Med. 2014. PMID: 24498626 Free PMC article.
-
Suppression of PAX6 promotes cell proliferation and inhibits apoptosis in human retinoblastoma cells.Int J Mol Med. 2014 Aug;34(2):399-408. doi: 10.3892/ijmm.2014.1812. Epub 2014 Jun 17. Int J Mol Med. 2014. PMID: 24939714 Free PMC article.
-
Identification of myopia-associated WNT7B polymorphisms provides insights into the mechanism underlying the development of myopia.Nat Commun. 2015 Mar 31;6:6689. doi: 10.1038/ncomms7689. Nat Commun. 2015. PMID: 25823570
-
Loss of δ-catenin function in severe autism.Nature. 2015 Apr 2;520(7545):51-6. doi: 10.1038/nature14186. Epub 2015 Mar 25. Nature. 2015. PMID: 25807484 Free PMC article.
References
-
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. - PubMed
-
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. - PubMed
-
- Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet. 2000;9:917–925. - PubMed
-
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. - PubMed
-
- Tang HK, Singh S, Saunders GF. Dissection of the transactivation function of the transcription factor encoded by the eye developmental gene PAX6. J Biol Chem. 1998;273:7210–7221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

