Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats?
- PMID: 20074611
- PMCID: PMC2859624
- DOI: 10.1016/j.pnpbp.2010.01.004
Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats?
Abstract
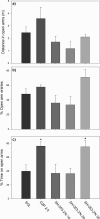
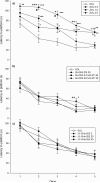
Over the last years, genetic studies have greatly improved our knowledge on the receptor subtypes mediating various pharmacological effects of positive allosteric modulators at GABA(A) receptors. This stimulated the development of new benzodiazepine (BZ)-like ligands, especially those inactive/low-active at GABA(A) receptors containing the alpha(1) subunit, with the aim of generating more selective drugs. Hereby, the affinity and efficacy of four recently synthesized BZ site ligands: SH-053-2'N, SH-053-S-CH3-2'F, SH-053-R-CH3-2'F and JY-XHe-053 were assessed. They were also studied in behavioral tests of spontaneous locomotor activity, elevated plus maze, and water maze in rats, which are considered predictive of, respectively, the sedative, anxiolytic, and amnesic influence of BZs. The novel ligands had moderately low to low affinity and mild to partial agonistic efficacy at GABA(A) receptors containing the alpha(1) subunit, with variable, but more pronounced efficacy at other BZ-sensitive binding sites. While presumably alpha(1) receptor-mediated sedative effects of GABA(A) modulation were not fully eliminated with any of the ligands tested, only SH-053-2'N and SH-053-S-CH3-2'F, both dosed at 30 mg/kg, exerted anxiolytic effects. The lack of clear anxiolytic-like activity of JY-XHe-053, despite its efficacy at alpha(2)- and alpha(3)-GABA(A) receptors, may have been partly connected with its preferential affinity at alpha(5)-GABA(A) receptors coupled with weak agonist activity at alpha(1)-containing subtypes. The memory impairment in water-maze experiments, generally reported with BZ site agonists, was completely circumvented with all four ligands. The results suggest that a substantial amount of activity at alpha(1) GABA(A) receptors is needed for affecting spatial learning and memory impairments, while much weaker activity at alpha(1)- and alpha(5)-GABA(A) receptors is sufficient for eliciting sedation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists?Neuropsychopharmacology. 2008 Jan;33(2):332-9. doi: 10.1038/sj.npp.1301403. Epub 2007 Mar 28. Neuropsychopharmacology. 2008. PMID: 17392731
-
PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats.Brain Res. 2008 May 7;1208:150-9. doi: 10.1016/j.brainres.2008.02.020. Epub 2008 Feb 19. Brain Res. 2008. PMID: 18394590 Free PMC article.
-
Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure.Neuropharmacology. 2010 Dec;59(7-8):612-8. doi: 10.1016/j.neuropharm.2010.08.011. Epub 2010 Aug 18. Neuropharmacology. 2010. PMID: 20727364 Free PMC article.
-
GABAA receptor subtype-selective modulators. II. α5-selective inverse agonists for cognition enhancement.Curr Top Med Chem. 2011;11(9):1203-14. doi: 10.2174/156802611795371314. Curr Top Med Chem. 2011. PMID: 21050171 Review.
-
Anxioselective compounds acting at the GABA(A) receptor benzodiazepine binding site.Curr Drug Targets CNS Neurol Disord. 2003 Aug;2(4):213-32. doi: 10.2174/1568007033482841. Curr Drug Targets CNS Neurol Disord. 2003. PMID: 12871032 Review.
Cited by
-
Nanocrystal dispersion of DK-I-56-1, a poorly soluble pyrazoloquinolinone positive modulator of α6 GABAA receptors: Formulation approach toward improved in vivo performance.Eur J Pharm Sci. 2020 Sep 1;152:105432. doi: 10.1016/j.ejps.2020.105432. Epub 2020 Jun 18. Eur J Pharm Sci. 2020. PMID: 32565331 Free PMC article.
-
Reductions in midbrain GABAergic and dopamine neuron markers are linked in schizophrenia.Mol Brain. 2021 Jun 26;14(1):96. doi: 10.1186/s13041-021-00805-7. Mol Brain. 2021. PMID: 34174930 Free PMC article.
-
Sex-Dependent Anti-Stress Effect of an α5 Subunit Containing GABAA Receptor Positive Allosteric Modulator.Front Pharmacol. 2016 Nov 22;7:446. doi: 10.3389/fphar.2016.00446. eCollection 2016. Front Pharmacol. 2016. PMID: 27920723 Free PMC article.
-
Benzodiazepines and Related Drugs as a Risk Factor in Alzheimer's Disease Dementia.Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344. eCollection 2019. Front Aging Neurosci. 2020. PMID: 31969812 Free PMC article.
-
Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia.Neuropharmacology. 2012 Mar;62(3):1342-8. doi: 10.1016/j.neuropharm.2011.05.011. Epub 2011 May 23. Neuropharmacology. 2012. PMID: 21621548 Free PMC article. Review.
References
-
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19:567–96. - PubMed
-
- Bandelow B, Zohar J, Hollander E, Kasper S, Möller HJ. WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disoders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders – first revision. World J Biol Psychiatry. 2008;9:248–312. - PubMed
-
- Cain DP. Testing the NMDA, long-term potentiation, and cholinergic hypotheses of spatial learning. Neurosci Biobehav Rev. 1998;22:181–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

