The lectin-like domain of TNF protects from listeriolysin-induced hyperpermeability in human pulmonary microvascular endothelial cells - a crucial role for protein kinase C-alpha inhibition
- PMID: 20074664
- PMCID: PMC3678561
- DOI: 10.1016/j.vph.2009.12.010
The lectin-like domain of TNF protects from listeriolysin-induced hyperpermeability in human pulmonary microvascular endothelial cells - a crucial role for protein kinase C-alpha inhibition
Abstract
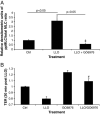
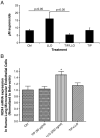
Listeriosis can lead to potentially lethal pulmonary complications in newborns and immune compromised patients, characterized by extensive permeability edema. Listeriolysin (LLO), the main virulence factor of Listeria monocytogenes, induces a dose-dependent hyperpermeability in monolayers of human lung microvascular endothelial cells in vitro. The permeability increasing activity of LLO, which is accompanied by an increased reactive oxygen species (ROS) generation, RhoA activation and myosin light chain (MLC) phosphorylation, can be completely inhibited by the protein kinase C (PKC) alpha/beta inhibitor GO6976, indicating a crucial role for PKC in the induction of barrier dysfunction. The TNF-derived TIP peptide, which mimics the lectin-like domain of the cytokine, blunts LLO-induced hyperpermeability in vitro, upon inhibiting LLO-induced protein kinase C-alpha activation, ROS generation and MLC phosphorylation and upon restoring the RhoA/Rac 1 balance. These results indicate that the lectin-like domain of TNF has a potential therapeutic value in protecting from LLO-induced pulmonary endothelial hyperpermeability.
Figures





Similar articles
-
Listeriolysin O Causes ENaC Dysfunction in Human Airway Epithelial Cells.Toxins (Basel). 2018 Feb 11;10(2):79. doi: 10.3390/toxins10020079. Toxins (Basel). 2018. PMID: 29439494 Free PMC article.
-
RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability.Am J Physiol Lung Cell Mol Physiol. 2005 Feb;288(2):L294-306. doi: 10.1152/ajplung.00213.2004. Epub 2004 Oct 8. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15475381
-
Human endothelial cell activation and mediator release in response to Listeria monocytogenes virulence factors.Infect Immun. 2001 Feb;69(2):897-905. doi: 10.1128/IAI.69.2.897-905.2001. Infect Immun. 2001. PMID: 11159983 Free PMC article.
-
Protein kinase C-α and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability.Am J Respir Cell Mol Biol. 2012 Oct;47(4):445-53. doi: 10.1165/rcmb.2011-0332OC. Epub 2012 May 10. Am J Respir Cell Mol Biol. 2012. PMID: 22582175 Free PMC article.
-
The relationship between Listeria infections and host immune responses: Listeriolysin O as a potential target.Biomed Pharmacother. 2024 Feb;171:116129. doi: 10.1016/j.biopha.2024.116129. Epub 2024 Jan 8. Biomed Pharmacother. 2024. PMID: 38194738 Review.
Cited by
-
TIP peptide inhalation in oleic acid-induced experimental lung injury: a post-hoc comparison.BMC Res Notes. 2013 Sep 27;6:385. doi: 10.1186/1756-0500-6-385. BMC Res Notes. 2013. PMID: 24070340 Free PMC article.
-
The pathogenesis and management of heatstroke and heatstroke-induced lung injury.Burns Trauma. 2025 Jan 14;13:tkae048. doi: 10.1093/burnst/tkae048. eCollection 2025. Burns Trauma. 2025. PMID: 39811431 Free PMC article. Review.
-
Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance.Front Physiol. 2022 Feb 21;12:793251. doi: 10.3389/fphys.2021.793251. eCollection 2021. Front Physiol. 2022. PMID: 35264975 Free PMC article. Review.
-
The SARS-CoV-2 spike S1 protein induces global proteomic changes in ATII-like rat L2 cells that are attenuated by hyaluronan.Am J Physiol Lung Cell Mol Physiol. 2023 Apr 1;324(4):L413-L432. doi: 10.1152/ajplung.00282.2022. Epub 2023 Jan 31. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 36719087 Free PMC article.
-
TNF Lectin-Like Domain Restores Epithelial Sodium Channel Function in Frameshift Mutants Associated with Pseudohypoaldosteronism Type 1B.Front Immunol. 2017 May 29;8:601. doi: 10.3389/fimmu.2017.00601. eCollection 2017. Front Immunol. 2017. PMID: 28611771 Free PMC article.
References
-
- Ananthraman A, Israel RH, Magnussen CR. Pleural-pulmonary aspects of Listeria monocytogenes infection. Respiration. 1983;44(2):153–157. - PubMed
-
- Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J. Cell Physiol. 2005;204(3):934–947. - PubMed
-
- Braun C, Hamacher J, Morel D, Wendel A, Lucas R. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J. Immunol. 2005;175(5):3402–3408. - PubMed
-
- Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J. Biol. Chem. 2006;281(3):1477–1488. - PubMed
-
- Darji A, Chakraborty T, Niebuhr K, Tsonis N, Wehland J, Weiss S. Hyperexpression of Listeriolysin in the non-pathogenic species Listeria innocua and high yield purification. J. Biotechnol. 1995;43(3):205–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

