Roles of Werner syndrome protein in protection of genome integrity
- PMID: 20075015
- PMCID: PMC2827637
- DOI: 10.1016/j.dnarep.2009.12.011
Roles of Werner syndrome protein in protection of genome integrity
Abstract
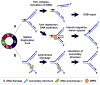
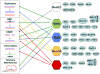
Werner syndrome protein (WRN) is one of a family of five human RecQ helicases implicated in the maintenance of genome stability. The conserved RecQ family also includes RecQ1, Bloom syndrome protein (BLM), RecQ4, and RecQ5 in humans, as well as Sgs1 in Saccharomyces cerevisiae, Rqh1 in Schizosaccharomyces pombe, and homologs in Caenorhabditis elegans, Xenopus laevis, and Drosophila melanogaster. Defects in three of the RecQ helicases, RecQ4, BLM, and WRN, cause human pathologies linked with cancer predisposition and premature aging. Mutations in the WRN gene are the causative factor of Werner syndrome (WS). WRN is one of the best characterized of the RecQ helicases and is known to have roles in DNA replication and repair, transcription, and telomere maintenance. Studies both in vitro and in vivo indicate that the roles of WRN in a variety of DNA processes are mediated by post-translational modifications, as well as several important protein-protein interactions. In this work, we will summarize some of the early studies on the cellular roles of WRN and highlight the recent findings that shed some light on the link between the protein with its cellular functions and the disease pathology.
(c) 2010 Elsevier B.V. All rights reserved.
Figures


References
-
- Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

