Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1
- PMID: 20075061
- PMCID: PMC2831854
- DOI: 10.1681/ASN.2009040421
Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1
Abstract
Aberrant activation of the mammalian target of rapamycin (mTOR) pathway occurs in polycystic kidney disease (PKD). mTOR inhibitors, such as rapamycin, are highly effective in several rodent models of PKD, but these models result from mutations in genes other than Pkd1 and Pkd2, which are the primary genes responsible for human autosomal dominant PKD. To address this limitation, we tested the efficacy of rapamycin in a mouse model that results from conditional inactivation of Pkd1. Mosaic deletion of Pkd1 resulted in PKD and replicated characteristic features of human PKD including aberrant mTOR activation, epithelial proliferation and apoptosis, and progressive fibrosis. Treatment with rapamycin was highly effective: It reduced cyst growth, preserved renal function, inhibited epithelial cell proliferation, increased apoptosis of cyst-lining cells, and inhibited fibrosis. These data provide in vivo evidence that rapamycin is effective in a human-orthologous mouse model of PKD.
Figures
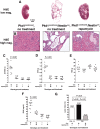

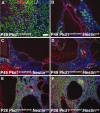

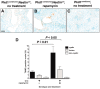

Comment in
-
Of mice and men: therapeutic mTOR inhibition in polycystic kidney disease.J Am Soc Nephrol. 2010 Mar;21(3):390-1. doi: 10.1681/ASN.2010010072. Epub 2010 Feb 4. J Am Soc Nephrol. 2010. PMID: 20133481 Free PMC article. No abstract available.
References
-
- Sutters M, Germino GG: Autosomal dominant polycystic kidney disease: Molecular genetics and pathophysiology. J Lab Clin Med 141: 91–101, 2003 - PubMed
-
- Ong AC, Harris PC: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 - PubMed
-
- Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 - PMC - PubMed
-
- Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, Patel V, Igarashi P, Alvord WG, Leighty R, Yao M, Bernardo M, Ileva L, Choyke P, Warren MB, Zbar B, Linehan WM, Schmidt LS: Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst 100: 140–154, 2008 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

