Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct
- PMID: 20075062
- PMCID: PMC2834543
- DOI: 10.1681/ASN.2009070728
Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct
Abstract
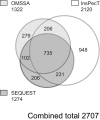
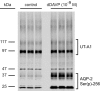
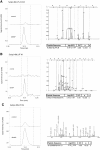
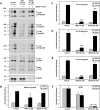
Protein phosphorylation is an important component of vasopressin signaling in the renal collecting duct, but the database of known phosphoproteins is incomplete. We used tandem mass spectrometry to identify vasopressin-regulated phosphorylation events in isolated rat inner medullary collecting duct (IMCD) suspensions. Using multiple search algorithms to identify the phosphopeptides from spectral data, we expanded the size of the existing collecting duct phosphoproteome database from 367 to 1187 entries. Label-free quantification in vasopressin- and vehicle-treated samples detected a significant change in the phosphorylation of 29 of 530 quantified phosphopeptides. The targets include important structural, regulatory, and transporter proteins. The vasopressin-regulated sites included two known sites (Ser-486 and Ser-499) present in the urea channel UT-A1 and one previously unknown site (Ser-84) on vasopressin-sensitive urea channels UT-A1 and UT-A3. In vitro assays using synthetic peptides showed that purified protein kinase A (PKA) could phosphorylate all three sites, and immunoblotting confirmed the PKA dependence of Ser-84 and Ser-486 phosphorylation. These results expand the known list of collecting duct phosphoproteins and highlight the utility of targeted phosphoproteomic approaches.
Figures





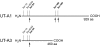
References
-
- Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney: From molecules to medicine. Physiol Rev 82: 205–244, 2002 - PubMed
-
- Brown D: The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003 - PubMed
-
- Yano Y, Rodrigues AC, Jr., de Braganca AC, Andrade LC, Magaldi AJ: PKC stimulated by glucagon decreases UT-A1 urea transporter expression in rat IMCD. Pflugers Arch 456: 1229–1237, 2008 - PubMed
-
- O'Connor PM, Cowley AW, Jr.: Vasopressin-induced nitric oxide production in rat inner medullary collecting duct is dependent on V2 receptor activation of the phosphoinositide pathway. Am J Physiol Renal Physiol 293: F526–F532, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

