Cross-talk between mitochondrial malate dehydrogenase and the cytochrome bc1 complex
- PMID: 20075069
- PMCID: PMC2856247
- DOI: 10.1074/jbc.M109.085787
Cross-talk between mitochondrial malate dehydrogenase and the cytochrome bc1 complex
Abstract
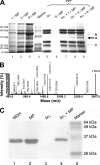
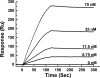
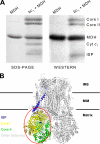
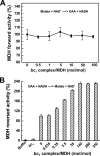
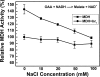
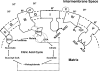
The interactions between the mitochondrial cytochrome bc(1) complex and matrix-soluble proteins were studied by a precipitation pulldown technique. Purified, detergent-dispersed bc(1) complex was incubated with mitochondrial matrix proteins followed by dialysis in the absence of detergent. The interacting protein(s) was co-precipitated with bc(1) complex upon centrifugation. One of the matrix proteins pulled down by bc(1) complex was identified as mitochondrial malate dehydrogenase (MDH) by matrix-assisted laser desorption ionization time-of-flight mass spectrometry and confirmed by Western blotting with anti-MDH antibody. Using a cross-linking technique, subunits I, II (core I and II), and V of the bc(1) complex were identified as the interacting sites for MDH. Incubating purified MDH with the detergent dispersed bc(1) complex results in an increase of the activities of both the bc(1) complex and MDH. The effect of the bc(1) complex on the activities of MDH is unidirectional (oxaloacetate --> malate). These results suggest that the novel cross-talk between citric acid cycle enzymes and electron transfer chain complexes might play a regulatory role in mitochondrial bioenergetics.
Figures

References
-
- Trumpower B. L., Gennis R. B. (1994) Annu. Rev. Biochem. 63, 675–716 - PubMed
-
- Iwata S., Lee J. W., Okada K., Lee J. K., Iwata M., Rasmussen B., Link T. A., Ramaswamy S., Jap B. K. (1998) Science 281, 64–71 - PubMed
-
- Berry E. A., Guergova-Kuras M., Huang L. S., Crofts A. R. (2000) Annu. Rev. Biochem. 69, 1005–1075 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

