International standards and reference materials for quantitative molecular infectious disease testing
- PMID: 20075208
- PMCID: PMC2871718
- DOI: 10.2353/jmoldx.2010.090067
International standards and reference materials for quantitative molecular infectious disease testing
Abstract
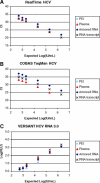
The utility of quantitative molecular diagnostics for patient management depends on the ability to relate patient results to prior results or to absolute values in clinical practice guidelines. To do this, those results need to be comparable across time and methods, either by producing the same value across methods and test versions or by using reliable and stable conversions. Universally available standards and reference materials specific to quantitative molecular technologies are critical to this process but are few in number. This review describes recent history in the establishment of international standards for nucleic acid test development, organizations involved in current efforts, and future issues and initiatives.
Figures
References
-
- Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. - PubMed
-
- O'Brien WA, Hartigan PM, Daar ES, Simberkoff MS, Hamilton JD. Changes in plasma HIV RNA levels and CD4+ counts predict both response to antiretroviral therapy and therapeutic failure: VA cooperative study group on AIDS. Ann Intern Med. 1997;126:983–985. - PubMed
-
- Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615–633. - PubMed
-
- Hammer SM, Saag MS, Schechter M, Montaner J, Schooley RT, Jacobsen DM, Thompson MA, Carpenter CCJ, Fischel MA, Gazzard BG, Gatell JM, Hirsch MS, Katzenstein DA, Richman DD, Vella S, Yeni PG, Volberding PA. Treatment for adult HIV infection. 2006 recommendations of the AIDS society—USA panel. JAMA. 2006;296:827–843. - PubMed
-
- Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H, Wright TL. A treatment algorithm for the management of chronic Hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006;4:936–962. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

