G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin "outside-in" signaling
- PMID: 20075254
- PMCID: PMC2842917
- DOI: 10.1126/science.1174779
G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin "outside-in" signaling
Abstract
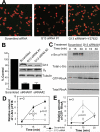
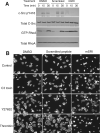
Integrins mediate cell adhesion to the extracellular matrix and transmit signals within the cell that stimulate cell spreading, retraction, migration, and proliferation. The mechanism of integrin outside-in signaling has been unclear. We found that the heterotrimeric guanine nucleotide-binding protein (G protein) Galpha13 directly bound to the integrin beta3 cytoplasmic domain and that Galpha13-integrin interaction was promoted by ligand binding to the integrin alphaIIbbeta3 and by guanosine triphosphate (GTP) loading of Galpha13. Interference of Galpha13 expression or a myristoylated fragment of Galpha13 that inhibited interaction of alphaIIbbeta3 with Galpha13 diminished activation of protein kinase c-Src and stimulated the small guanosine triphosphatase RhoA, consequently inhibiting cell spreading and accelerating cell retraction. We conclude that integrins are noncanonical Galpha13-coupled receptors that provide a mechanism for dynamic regulation of RhoA.
Figures


Comment in
-
Integrin αIIbβ3: a novel effector of Gα13.Cell Adh Migr. 2011 Jan-Feb;5(1):4-5. doi: 10.4161/cam.5.1.13559. Epub 2011 Jan 1. Cell Adh Migr. 2011. PMID: 20930548 Free PMC article.
Similar articles
-
PPARγ agonists negatively regulate αIIbβ3 integrin outside-in signaling and platelet function through up-regulation of protein kinase A activity.J Thromb Haemost. 2017 Feb;15(2):356-369. doi: 10.1111/jth.13578. Epub 2017 Feb 7. J Thromb Haemost. 2017. PMID: 27896950 Free PMC article.
-
The interaction of Gα13 with integrin β1 mediates cell migration by dynamic regulation of RhoA.Mol Biol Cell. 2015 Oct 15;26(20):3658-70. doi: 10.1091/mbc.E15-05-0274. Epub 2015 Aug 26. Mol Biol Cell. 2015. PMID: 26310447 Free PMC article.
-
A directional switch of integrin signalling and a new anti-thrombotic strategy.Nature. 2013 Nov 7;503(7474):131-5. doi: 10.1038/nature12613. Epub 2013 Oct 27. Nature. 2013. PMID: 24162846 Free PMC article.
-
Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction.Curr Opin Cell Biol. 2012 Oct;24(5):600-6. doi: 10.1016/j.ceb.2012.08.011. Epub 2012 Sep 11. Curr Opin Cell Biol. 2012. PMID: 22980731 Free PMC article. Review.
-
Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review.
Cited by
-
Exogenous Integrin αIIbβ3 Inhibitors Revisited: Past, Present and Future Applications.Int J Mol Sci. 2021 Mar 25;22(7):3366. doi: 10.3390/ijms22073366. Int J Mol Sci. 2021. PMID: 33806083 Free PMC article. Review.
-
Gα13 Switch Region 2 Relieves Talin Autoinhibition to Activate αIIbβ3 Integrin.J Biol Chem. 2016 Dec 23;291(52):26598-26612. doi: 10.1074/jbc.M116.747279. Epub 2016 Nov 1. J Biol Chem. 2016. PMID: 27803165 Free PMC article.
-
The Ric-8A/Gα13/FAK signalling cascade controls focal adhesion formation during neural crest cell migration in Xenopus.Development. 2018 Nov 21;145(22):dev164269. doi: 10.1242/dev.164269. Development. 2018. PMID: 30297374 Free PMC article.
-
From Discovery of Snake Venom Disintegrins to A Safer Therapeutic Antithrombotic Agent.Toxins (Basel). 2019 Jun 26;11(7):372. doi: 10.3390/toxins11070372. Toxins (Basel). 2019. PMID: 31247995 Free PMC article. Review.
-
Integrin activation is an essential component of SARS-CoV-2 infection.Sci Rep. 2021 Oct 14;11(1):20398. doi: 10.1038/s41598-021-99893-7. Sci Rep. 2021. PMID: 34650161 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

