Leading the way: canine models of genomics and disease
- PMID: 20075379
- PMCID: PMC4068608
- DOI: 10.1242/dmm.004358
Leading the way: canine models of genomics and disease
Abstract
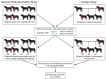
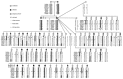
In recent years Canis familiaris, the domestic dog, has drawn considerable attention as a system in which to investigate the genetics of disease susceptibility, morphology and behavior. Because dogs show remarkable intrabreed homogeneity, coupled with striking interbreed heterogeneity, the dog offers unique opportunities to understand the genetic underpinnings of natural variation in mammals, a portion of which is disease susceptibility. In this review, we highlight the unique features of the dog, such as population diversity and breed structure, that make it particularly amenable to genetic studies. We highlight recent advances in understanding the architecture of the dog genome, which propel the system to the forefront of consideration when selecting a system for disease gene studies. The most notable benefit of using the dog for genetic studies is that dogs get many of the same diseases as humans, with a similar frequency, and the same genetic factors are often involved. We discuss two approaches for localizing disease genes in the dog and provide examples of ongoing studies.
Figures
References
-
- Acland GM, Ray K, Mellersh CS, Gu W, Langston AA, Rine J, Ostrander EA, Aguirre GD. (1998). Linkage analysis and comparative mapping of canine progressive rod-cone degeneration (prcd) establishes potential locus homology with retinitis pigmentosa (RP17) in humans. Proc Natl Acad Sci USA 96, 3048–3053 - PMC - PubMed
-
- Acland GM, Ray K, Mellersh CS, Langston AA, Rine J, Ostrander EA, Aguirre GD. (1999). A novel retinal degeneration locus identified by linkage and comparative mapping of canine early retinal degeneration. Genomics 59, 134–142 - PubMed
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. (2001). Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 28, 92–95 - PubMed
-
- Affolter VK, Moore PF. (2000). Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. Am J Dermatopathol. 22, 40–48 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

