Vitamin D levels, lung function, and steroid response in adult asthma
- PMID: 20075384
- PMCID: PMC2868500
- DOI: 10.1164/rccm.200911-1710OC
Vitamin D levels, lung function, and steroid response in adult asthma
Abstract
Rationale: Patients with asthma exhibit variable response to inhaled corticosteroids (ICS). Vitamin D is hypothesized to exert effects on phenotype and glucocorticoid (GC) response in asthma.
Objectives: To determine the effect of vitamin D levels on phenotype and GC response in asthma.
Methods: Nonsmoking adults with asthma were enrolled in a study assessing the relationship between serum 25(OH)D (vitamin D) concentrations and lung function, airway hyperresponsiveness (AHR), and GC response, as measured by dexamethasone-induced expression of mitogen-activated protein kinase phosphatase (MKP)-1 by peripheral blood mononuclear cells.
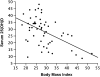
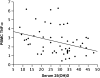
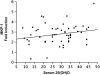
Measurements and main results: A total of 54 adults with asthma (FEV(1), 82.9 +/- 15.7% predicted [mean +/- SD], serum vitamin D levels of 28.1 +/- 10.2 ng/ml) were enrolled. Higher vitamin D levels were associated with greater lung function, with a 22.7 (+/-9.3) ml (mean +/- SE) increase in FEV(1) for each nanogram per milliliter increase in vitamin D (P = 0.02). Participants with vitamin D insufficiency (<30 ng/ml) demonstrated increased AHR, with a provocative concentration of methacholine inducing a 20% fall in FEV(1) of 1.03 (+/-0.2) mg/ml versus 1.92 (+/-0.2) mg/ml in those with vitamin D of 30 ng/ml or higher (P = 0.01). In ICS-untreated participants, dexamethasone-induced MKP-1 expression increased with higher vitamin D levels, with a 0.05 (+/-0.02)-fold increase (P = 0.02) in MKP-1 expression observed for each nanogram per milliliter increase in vitamin D, a finding that occurred in the absence of a significant increase in IL-10 expression.
Conclusions: In asthma, reduced vitamin D levels are associated with impaired lung function, increased AHR, and reduced GC response, suggesting that supplementation of vitamin D levels in patients with asthma may improve multiple parameters of asthma severity and treatment response. Clinical trials registered with www.clinicaltrials.gov (NCT00495157, NCT00565266, and NCT00557180).
Figures


References
-
- U.S. Department of Health and Human Services. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program; 2007. NIH Publication No. 07-4051.
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention: 2008 update. Bethesda, MD: National Heart, Lung, and Blood Institute [updated 2009; accessed November, 2009]. Available from: http://www.ginasthma.org/guidelineitem.asp??l1=2&l2=1&intId=1561.
-
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836–844. - PubMed
-
- Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest 2008;134:394–401. - PubMed
-
- Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487–495. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

