P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes
- PMID: 20075394
- PMCID: PMC2857638
- DOI: 10.1095/biolreprod.109.082057
P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes
Abstract
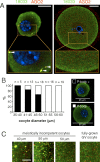
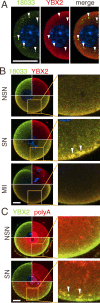
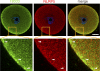
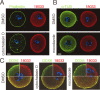
In mammalian somatic cells, several pathways that converge on deadenylation, decapping, and 5'-3' degradation are found in cytoplasmic foci known as P-bodies. Because controlled mRNA stability is essential for oocyte-to-zygote transition, we examined the dynamics of P-body components in mouse oocytes. We report that oocyte growth is accompanied by loss of P-bodies and a subcortical accumulation of several RNA-binding proteins, including DDX6, CPEB, YBX2 (MSY2), and the exon junction complex. These proteins form transient RNA-containing aggregates in fully grown oocytes with a surrounded nucleolus chromatin configuration. These aggregates disperse during oocyte maturation, consistent with recruitment of maternal mRNAs that occurs during this time. In contrast, levels of DCP1A are low during oocyte growth, and DCP1A does not colocalize with DDX6 in the subcortical aggregates. The amount of DCP1A markedly increases during meiosis, which correlates with the first wave of destabilization of maternal mRNAs. We propose that the cortex of growing oocytes serves as an mRNA storage compartment, which contains a novel type of RNA granule related to P-bodies.
Figures


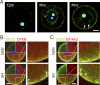
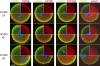
Comment in
-
A novel maternal mRNA storage compartment in mouse oocytes.Biol Reprod. 2010 May;82(5):807-8. doi: 10.1095/biolreprod.110.084376. Epub 2010 Mar 10. Biol Reprod. 2010. PMID: 20220128 No abstract available.
References
-
- Brower PT, Gizang E, Boreen SM, Schultz RM.Biochemical studies of mammalian oogenesis: synthesis and stability of various classes of RNA during growth of the mouse oocyte in vitro. Dev Biol 1981; 86: 373–383. - PubMed
-
- Richter JD.CPEB: a life in translation. Trends Biochem Sci 2007; 32: 279–285. - PubMed
-
- Eulalio A, Behm-Ansmant I, Izaurralde E.P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol 2007; 8: 9–22. - PubMed
-
- Parker R, Sheth U.P bodies and the control of mRNA translation and degradation. Mol Cell 2007; 25: 635–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

