High basal cell surface levels of fish GLUT4 are related to reduced sensitivity of insulin-induced translocation toward GGA and AS160 inhibition in adipocytes
- PMID: 20075431
- PMCID: PMC2822488
- DOI: 10.1152/ajpendo.00547.2009
High basal cell surface levels of fish GLUT4 are related to reduced sensitivity of insulin-induced translocation toward GGA and AS160 inhibition in adipocytes
Abstract
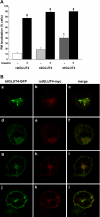
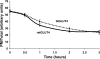
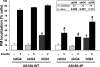
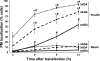
Glucose entry into cells is mediated by a family of facilitative transporter proteins (GLUTs). In mammals, GLUT4 is expressed in insulin-sensitive tissues and is responsible for the postprandial uptake of glucose. In fish, GLUT4 also mediates insulin-regulated glucose entry into cells but differs from mammalian GLUT4 in its affinity for glucose and in protein motifs known to be important for the traffic of GLUT4. In this study, we have characterized the intracellular and plasma membrane (PM) traffic of two orthologs of GLUT4 in fish, trout (btGLUT4) and salmon (okGLUT4), that do not share the amino terminal FQQI targeting motif of mammalian GLUT4. btGLUT4 (FQHL) and, to a lesser extent, okGLUT4 (FQQL) showed higher basal PM levels, faster traffic to the PM after biosynthesis, and earlier acquisition of insulin responsiveness than rat GLUT4. Furthermore, btGLUT4 showed a similar profile of internalization than rat GLUT4. Expression of the dominant-interfering AS160-4P mutant caused a significant decrease in the insulin-induced PM levels of okGLUT4 and rat GLUT4 and, to a lesser extent, of btGLUT4, suggesting that btGLUT4 has reduced retention into the IRC. Contrary to rat GLUT4 and okGLUT4, the presence of btGLUT4 at the PM under insulin-stimulated conditions was not affected by coexpression of a dominant-interfering GGA mutant. These data suggest that fish GLUT4 follow a different trafficking pathway to the PM compared with rat GLUT4 that seems to be relatively independent of GGA. These results indicate that the regulated trafficking characteristics of GLUT4 have been modified during evolution from fish to mammals.
Figures
Similar articles
-
Fish glucose transporter (GLUT)-4 differs from rat GLUT4 in its traffic characteristics but can translocate to the cell surface in response to insulin in skeletal muscle cells.Endocrinology. 2007 Nov;148(11):5248-57. doi: 10.1210/en.2007-0265. Epub 2007 Aug 16. Endocrinology. 2007. PMID: 17702851
-
Functional characterization of an insulin-responsive glucose transporter (GLUT4) from fish adipose tissue.Am J Physiol Endocrinol Metab. 2004 Aug;287(2):E348-57. doi: 10.1152/ajpendo.00538.2003. Epub 2004 Apr 27. Am J Physiol Endocrinol Metab. 2004. PMID: 15113704
-
The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment.Mol Endocrinol. 2007 Dec;21(12):3087-99. doi: 10.1210/me.2006-0476. Epub 2007 Aug 30. Mol Endocrinol. 2007. PMID: 17761952
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
-
Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners.Endocrinology. 2005 Dec;146(12):5071-8. doi: 10.1210/en.2005-0850. Epub 2005 Sep 8. Endocrinology. 2005. PMID: 16150904 Review.
Cited by
-
Mechanisms regulating GLUT4 transcription in skeletal muscle cells are highly conserved across vertebrates.PLoS One. 2013 Nov 18;8(11):e80628. doi: 10.1371/journal.pone.0080628. eCollection 2013. PLoS One. 2013. PMID: 24260440 Free PMC article.
References
-
- Al-Hasani H, Kunamneni RK, Dawson K, Hinck CS, Müller-Wieland D, Cushman SW. Roles of the N- and C-termini of GLUT4 in endocytosis. J Cell Sci 115: 131–140, 2002 - PubMed
-
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3: 267–277, 2002 - PubMed
-
- Capilla E, Díaz M, Albalat A, Navarro I, Pessin JE, Keller K, Planas JV. Functional characterization of an insulin-responsive glucose transporter (GLUT4) from fish adipose tissue. Am J Physiol Endocrinol Metab 287: E348–E357, 2004 - PubMed
-
- Capilla E, Suzuki N, Pessin JE, Hou JC. The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment. Mol Endocrinol 21: 3087–3099, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

