Eukaryotic ribonucleases P/MRP: the crystal structure of the P3 domain
- PMID: 20075859
- PMCID: PMC2829168
- DOI: 10.1038/emboj.2009.396
Eukaryotic ribonucleases P/MRP: the crystal structure of the P3 domain
Abstract
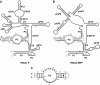
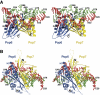
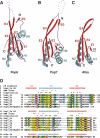
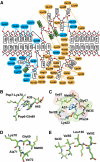
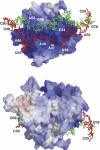
Ribonuclease (RNase) P is a site-specific endoribonuclease found in all kingdoms of life. Typical RNase P consists of a catalytic RNA component and a protein moiety. In the eukaryotes, the RNase P lineage has split into two, giving rise to a closely related enzyme, RNase MRP, which has similar components but has evolved to have different specificities. The eukaryotic RNases P/MRP have acquired an essential helix-loop-helix protein-binding RNA domain P3 that has an important function in eukaryotic enzymes and distinguishes them from bacterial and archaeal RNases P. Here, we present a crystal structure of the P3 RNA domain from Saccharomyces cerevisiae RNase MRP in a complex with RNase P/MRP proteins Pop6 and Pop7 solved to 2.7 A. The structure suggests similar structural organization of the P3 RNA domains in RNases P/MRP and possible functions of the P3 domains and proteins bound to them in the stabilization of the holoenzymes' structures as well as in interactions with substrates. It provides the first insight into the structural organization of the eukaryotic enzymes of the RNase P/MRP family.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Specific binding of a Pop6/Pop7 heterodimer to the P3 stem of the yeast RNase MRP and RNase P RNAs.RNA. 2007 Oct;13(10):1648-55. doi: 10.1261/rna.654407. Epub 2007 Aug 23. RNA. 2007. PMID: 17717080 Free PMC article.
-
Footprinting analysis of interactions between the largest eukaryotic RNase P/MRP protein Pop1 and RNase P/MRP RNA components.RNA. 2015 Sep;21(9):1591-605. doi: 10.1261/rna.049007.114. Epub 2015 Jul 1. RNA. 2015. PMID: 26135751 Free PMC article.
-
Functional equivalence of hairpins in the RNA subunits of RNase MRP and RNase P in Saccharomyces cerevisiae.RNA. 2000 May;6(5):653-8. doi: 10.1017/s1355838200992574. RNA. 2000. PMID: 10836786 Free PMC article.
-
Of proteins and RNA: the RNase P/MRP family.RNA. 2010 Sep;16(9):1725-47. doi: 10.1261/rna.2214510. Epub 2010 Jul 13. RNA. 2010. PMID: 20627997 Free PMC article. Review.
-
Structural and functional similarities between MRP and RNase P.Mol Biol Rep. 1995-1996;22(2-3):81-5. doi: 10.1007/BF00988710. Mol Biol Rep. 1995. PMID: 8901492 Review.
Cited by
-
RNA binding properties of conserved protein subunits of human RNase P.Nucleic Acids Res. 2011 Jul;39(13):5704-14. doi: 10.1093/nar/gkr126. Epub 2011 Mar 30. Nucleic Acids Res. 2011. PMID: 21450806 Free PMC article.
-
Cooperative RNP assembly: complementary rescue of structural defects by protein and RNA subunits of archaeal RNase P.J Mol Biol. 2011 Aug 12;411(2):368-83. doi: 10.1016/j.jmb.2011.05.012. Epub 2011 Jun 12. J Mol Biol. 2011. PMID: 21683084 Free PMC article.
-
Structural organizations of yeast RNase P and RNase MRP holoenzymes as revealed by UV-crosslinking studies of RNA-protein interactions.RNA. 2012 Apr;18(4):720-8. doi: 10.1261/rna.030874.111. Epub 2012 Feb 13. RNA. 2012. PMID: 22332141 Free PMC article.
-
RNase MRP cleaves pre-tRNASer-Met in the tRNA maturation pathway.PLoS One. 2014 Nov 17;9(11):e112488. doi: 10.1371/journal.pone.0112488. eCollection 2014. PLoS One. 2014. PMID: 25401760 Free PMC article.
-
Small Molecule with Big Impact: Metarrestin Targets the Perinucleolar Compartment in Cancer Metastasis.Cells. 2024 Dec 12;13(24):2053. doi: 10.3390/cells13242053. Cells. 2024. PMID: 39768145 Free PMC article. Review.
References
-
- Altman S, Kirsebom L (1999) Ribonuclease P. In The RNA World, Gesteland RF, Cech TR, Alkins JF (eds), pp 351–380. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press
-
- Brunger AT, Adams PD, Clore GM, Delano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges N, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography and NMR system (CNS): a new software system for macromolecular structure determination. Acta Cryst D 54: 905–921 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

