Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway
- PMID: 20075862
- PMCID: PMC2837171
- DOI: 10.1038/emboj.2009.407
Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway
Abstract
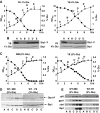
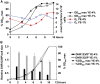
Either calorie restriction, loss-of-function of the nutrient-dependent PKA or TOR/SCH9 pathways, or activation of stress defences improves longevity in different eukaryotes. However, the molecular links between glucose depletion, nutrient-dependent pathways and stress responses are unknown. Here, we show that either calorie restriction or inactivation of nutrient-dependent pathways induces lifespan extension in fission yeast, and that such effect is dependent on the activation of the stress-dependent Sty1 mitogen-activated protein (MAP) kinase. During transition to stationary phase in glucose-limiting conditions, Sty1 becomes activated and triggers a transcriptional stress programme, whereas such activation does not occur under glucose-rich conditions. Deletion of the genes coding for the SCH9-homologue, Sck2 or the Pka1 kinases, or mutations leading to constitutive activation of the Sty1 stress pathway increase lifespan under glucose-rich conditions, and importantly such beneficial effects depend ultimately on Sty1. Furthermore, cells lacking Pka1 display enhanced oxygen consumption and Sty1 activation under glucose-rich conditions. We conclude that calorie restriction favours oxidative metabolism, reactive oxygen species production and Sty1 MAP kinase activation, and this stress pathway favours lifespan extension.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



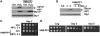


Similar articles
-
Deciphering the role of the signal- and Sty1 kinase-dependent phosphorylation of the stress-responsive transcription factor Atf1 on gene activation.J Biol Chem. 2017 Aug 18;292(33):13635-13644. doi: 10.1074/jbc.M117.794339. Epub 2017 Jun 26. J Biol Chem. 2017. PMID: 28652406 Free PMC article.
-
The roles of stress-activated Sty1 and Gcn2 kinases and of the protooncoprotein homologue Int6/eIF3e in responses to endogenous oxidative stress during histidine starvation.J Mol Biol. 2010 Nov 26;404(2):183-201. doi: 10.1016/j.jmb.2010.09.016. Epub 2010 Sep 25. J Mol Biol. 2010. PMID: 20875427 Free PMC article.
-
Transcription factors Atf1 and Sty1 promote stress tolerance under nitrosative stress in Schizosaccharomyces pombe.Microbiol Res. 2018 Jan;206:82-90. doi: 10.1016/j.micres.2017.10.002. Epub 2017 Oct 12. Microbiol Res. 2018. PMID: 29146263
-
Nuclear roles and regulation of chromatin structure by the stress-dependent MAP kinase Sty1 of Schizosaccharomyces pombe.Mol Microbiol. 2011 Nov;82(3):542-54. doi: 10.1111/j.1365-2958.2011.07851.x. Epub 2011 Oct 13. Mol Microbiol. 2011. PMID: 21992435 Review.
-
Phospho-mimicking Atf1 mutants bypass the transcription activating function of the MAP kinase Sty1 of fission yeast.Curr Genet. 2018 Feb;64(1):97-102. doi: 10.1007/s00294-017-0730-7. Epub 2017 Aug 10. Curr Genet. 2018. PMID: 28799013 Review.
Cited by
-
Genes affecting the extension of chronological lifespan in Schizosaccharomyces pombe (fission yeast).Mol Microbiol. 2021 Apr;115(4):623-642. doi: 10.1111/mmi.14627. Epub 2020 Nov 3. Mol Microbiol. 2021. PMID: 33064911 Free PMC article. Review.
-
Sestrin2, a Regulator of Thermogenesis and Mitohormesis in Brown Adipose Tissue.Front Endocrinol (Lausanne). 2015 Jul 24;6:114. doi: 10.3389/fendo.2015.00114. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26257706 Free PMC article. Review.
-
Antagonistic effects of mitochondrial matrix and intermembrane space proteases on yeast aging.BMC Biol. 2022 Jul 12;20(1):160. doi: 10.1186/s12915-022-01352-w. BMC Biol. 2022. PMID: 35820914 Free PMC article.
-
Nutrient Limitation Inactivates Mrc1-to-Cds1 Checkpoint Signalling in Schizosaccharomyces pombe.Cells. 2018 Feb 23;7(2):15. doi: 10.3390/cells7020015. Cells. 2018. PMID: 29473861 Free PMC article.
-
Glucose controls phosphoregulation of hydroxymethylglutaryl coenzyme A reductase through the protein phosphatase 2A-related phosphatase protein, Ppe1, and Insig in fission yeast.J Biol Chem. 2011 Aug 5;286(31):27139-46. doi: 10.1074/jbc.M111.233452. Epub 2011 Jun 16. J Biol Chem. 2011. PMID: 21680738 Free PMC article.
References
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E (1993) Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory
-
- Bishop NA, Guarente L (2007) Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet 8: 835–844 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

