A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses
- PMID: 20075863
- PMCID: PMC2837166
- DOI: 10.1038/emboj.2009.400
A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses
Abstract
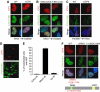
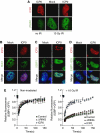
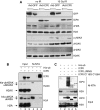
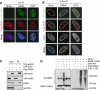
The ICP0 protein of herpes simplex virus type 1 is an E3 ubiquitin ligase and transactivator required for the efficient switch between latent and lytic infection. As DNA damaging treatments are known to reactivate latent virus, we wished to explore whether ICP0 modulates the cellular response to DNA damage. We report that ICP0 prevents accumulation of repair factors at cellular damage sites, acting between recruitment of the mediator proteins Mdc1 and 53BP1. We identify RNF8 and RNF168, cellular histone ubiquitin ligases responsible for anchoring repair factors at sites of damage, as new targets for ICP0-mediated degradation. By targeting these ligases, ICP0 expression results in loss of ubiquitinated forms of H2A, mobilization of DNA repair proteins and enhanced viral fitness. Our study raises the possibility that the ICP0-mediated control of histone ubiquitination may link DNA repair, relief of transcriptional repression, and activation of latent viral genomes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain.Mol Cell. 2012 Apr 13;46(1):79-90. doi: 10.1016/j.molcel.2012.02.004. Epub 2012 Mar 7. Mol Cell. 2012. PMID: 22405594 Free PMC article.
-
The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0.PLoS Pathog. 2011 Jun;7(6):e1002084. doi: 10.1371/journal.ppat.1002084. Epub 2011 Jun 16. PLoS Pathog. 2011. PMID: 21698222 Free PMC article.
-
Ubiquitin-H2AX fusions render 53BP1 recruitment to DNA damage sites independent of RNF8 or RNF168.Cell Cycle. 2015;14(11):1748-58. doi: 10.1080/15384101.2015.1010918. Cell Cycle. 2015. PMID: 25695757 Free PMC article.
-
Identification of a novel higher molecular weight isoform of USP7/HAUSP that interacts with the Herpes simplex virus type-1 immediate early protein ICP0.Virus Res. 2008 Oct;137(1):64-71. doi: 10.1016/j.virusres.2008.05.017. Epub 2008 Jul 17. Virus Res. 2008. PMID: 18590780
-
The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection.Virus Res. 2020 Aug;285:198015. doi: 10.1016/j.virusres.2020.198015. Epub 2020 May 13. Virus Res. 2020. PMID: 32416261 Free PMC article. Review.
Cited by
-
N-terminal phosphorylation sites of herpes simplex virus 1 ICP0 differentially regulate its activities and enhance viral replication.J Virol. 2013 Feb;87(4):2109-19. doi: 10.1128/JVI.02588-12. Epub 2012 Dec 5. J Virol. 2013. PMID: 23221554 Free PMC article.
-
Chromatin dynamics during lytic infection with herpes simplex virus 1.Viruses. 2013 Jul 16;5(7):1758-86. doi: 10.3390/v5071758. Viruses. 2013. PMID: 23863878 Free PMC article. Review.
-
Neighborly DISCourse: DNA double strand breaks silence transcription.Cell Cycle. 2010 Sep 15;9(18):3635-6. doi: 10.4161/cc.9.18.13171. Epub 2010 Sep 28. Cell Cycle. 2010. PMID: 20855969 Free PMC article. No abstract available.
-
Human papillomavirus E7 oncoprotein targets RNF168 to hijack the host DNA damage response.Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19552-19562. doi: 10.1073/pnas.1906102116. Epub 2019 Sep 9. Proc Natl Acad Sci U S A. 2019. PMID: 31501315 Free PMC article.
-
Core histones H2B and H4 are mobilized during infection with herpes simplex virus 1.J Virol. 2011 Dec;85(24):13234-52. doi: 10.1128/JVI.06038-11. Epub 2011 Oct 12. J Virol. 2011. PMID: 21994445 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

