Granzyme B cleavage of autoantigens in autoimmunity
- PMID: 20075942
- PMCID: PMC3136751
- DOI: 10.1038/cdd.2009.197
Granzyme B cleavage of autoantigens in autoimmunity
Abstract
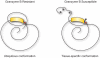
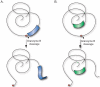
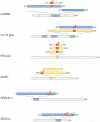
The systemic autoimmune diseases are a complex group of disorders characterized by elaboration of high titer autoantibodies and immune-mediated damage of tissues. Two striking features of autoimmune rheumatic diseases are their self-sustaining nature and capacity for autoamplification, exemplified by disease flares. These features suggest the presence of a feed-forward cycle in disease propagation, in which immune effector pathways drive the generation/release of autoantigens, which in turn fuel the immune response. There is a growing awareness that structural modification during cytotoxic granule-induced cell death is a frequent and striking feature of autoantigens, and may be an important principle driving disease. This review focuses on granzyme B (GrB)-mediated cleavage of autoantigens including (i) features of GrB cleavage sites within autoantigens, (ii) co-location of cleavage sites with autoimmune epitopes, and (iii) GrB sensitivity of autoantigens in disease-relevant target tissue. The mechanisms whereby GrB-induced changes in autoantigen structure may contribute to the initiation and propagation of autoimmunity are reviewed and reveal that GrB has the potential to create or destroy autoimmune epitopes. As there remains no direct evidence showing a causal function for GrB cleavage of antigens in the generation of autoimmunity, this review highlights important outstanding questions about the function of GrB in autoantigen selection.
Figures

Similar articles
-
Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity.J Exp Med. 1999 Sep 20;190(6):815-26. doi: 10.1084/jem.190.6.815. J Exp Med. 1999. PMID: 10499920 Free PMC article.
-
Cleavage of transaldolase by granzyme B causes the loss of enzymatic activity with retention of antigenicity for multiple sclerosis patients.J Immunol. 2010 Apr 1;184(7):4025-32. doi: 10.4049/jimmunol.0804174. Epub 2010 Mar 1. J Immunol. 2010. PMID: 20194725 Free PMC article.
-
Proteolysis by Granzyme B Enhances Presentation of Autoantigenic Peptidylarginine Deiminase 4 Epitopes in Rheumatoid Arthritis.J Proteome Res. 2017 Jan 6;16(1):355-365. doi: 10.1021/acs.jproteome.6b00617. Epub 2016 Oct 20. J Proteome Res. 2017. PMID: 27700100 Free PMC article.
-
Autoantigens as Partners in Initiation and Propagation of Autoimmune Rheumatic Diseases.Annu Rev Immunol. 2016 May 20;34:395-420. doi: 10.1146/annurev-immunol-032414-112205. Epub 2016 Feb 22. Annu Rev Immunol. 2016. PMID: 26907212 Free PMC article. Review.
-
Altered autoantigen structure in Sjögren's syndrome: implications for the pathogenesis of autoimmune tissue damage.Crit Rev Oral Biol Med. 2004 Jun 4;15(3):156-64. doi: 10.1177/154411130401500304. Crit Rev Oral Biol Med. 2004. PMID: 15187033 Review.
Cited by
-
Gene Expression Meta-Analysis Reveals Concordance in Gene Activation, Pathway, and Cell-Type Enrichment in Dermatomyositis Target Tissues.ACR Open Rheumatol. 2019 Nov 9;1(10):657-666. doi: 10.1002/acr2.11081. eCollection 2019 Dec. ACR Open Rheumatol. 2019. PMID: 31872188 Free PMC article.
-
Risk of generalized vitiligo is associated with the common 55R-94A-247H variant haplotype of GZMB (encoding granzyme B).J Invest Dermatol. 2013 Jun;133(6):1677-9. doi: 10.1038/jid.2013.5. Epub 2013 Jan 15. J Invest Dermatol. 2013. PMID: 23321921 Free PMC article.
-
Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin.PLoS One. 2012;7(12):e51040. doi: 10.1371/journal.pone.0051040. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251420 Free PMC article.
-
Cytotoxic B Cells in Relapsing-Remitting Multiple Sclerosis Patients.Front Immunol. 2022 Feb 7;13:750660. doi: 10.3389/fimmu.2022.750660. eCollection 2022. Front Immunol. 2022. PMID: 35197967 Free PMC article.
-
Modulatory function of invariant natural killer T cells in systemic lupus erythematosus.Clin Dev Immunol. 2012;2012:478429. doi: 10.1155/2012/478429. Epub 2012 Jun 13. Clin Dev Immunol. 2012. PMID: 22761630 Free PMC article. Review.
References
-
- Gabrielli A, Svegliati S, Moroncini G, Avvedimento EV. Pathogenic autoantibodies in systemic sclerosis. Curr.Opin.Immunol. 2007 Dec;19(6):640–645. - PubMed
-
- Gunawardena H, Betteridge ZE, McHugh NJ. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 2009 Jun;48(6):607–612. - PubMed
-
- Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005 Feb;38(1):47–54. - PubMed
-
- Rodriguez-Reyna TS, Alarcon-Segovia D. Overlap syndromes in the context of shared autoimmunity. Autoimmunity. 2005 May;38(3):219–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

