Regulation of apoptosis-associated lysosomal membrane permeabilization
- PMID: 20077016
- PMCID: PMC2850995
- DOI: 10.1007/s10495-009-0452-5
Regulation of apoptosis-associated lysosomal membrane permeabilization
Abstract
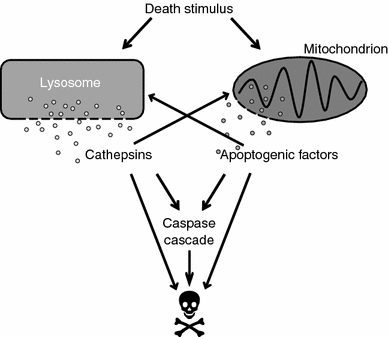
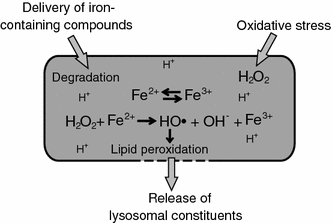
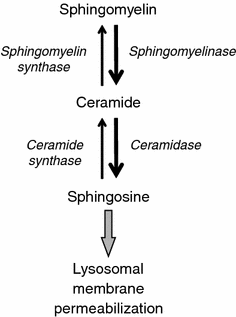
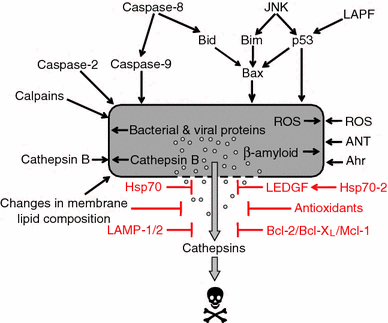
Lysosomal membrane permeabilization (LMP) occurs in response to a large variety of cell death stimuli causing release of cathepsins from the lysosomal lumen into the cytosol where they participate in apoptosis signaling. In some settings, apoptosis induction is dependent on an early release of cathepsins, while under other circumstances LMP occurs late in the cell death process and contributes to amplification of the death signal. The mechanism underlying LMP is still incompletely understood; however, a growing body of evidence suggests that LMP may be governed by several distinct mechanisms that are likely engaged in a death stimulus- and cell-type-dependent fashion. In this review, factors contributing to permeabilization of the lysosomal membrane including reactive oxygen species, lysosomal membrane lipid composition, proteases, p53, and Bcl-2 family proteins, are described. Potential mechanisms to safeguard lysosomal integrity and confer resistance to lysosome-dependent cell death are also discussed.
Figures

References
-
- de Duve C. Lysosomes, a new group of cytoplasmic particles. In: Hayashi T, editor. Subcellular particles. New York: Ronald Press; 1959. pp. 128–159.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

