Glycoprotein analysis using protein microarrays and mass spectrometry
- PMID: 20077480
- PMCID: PMC2889184
- DOI: 10.1002/mas.20269
Glycoprotein analysis using protein microarrays and mass spectrometry
Abstract
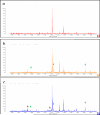
Protein glycosylation plays an important role in a multitude of biological processes such as cell-cell recognition, growth, differentiation, and cell death. It has been shown that specific glycosylation changes are key in disease progression and can have diagnostic value for a variety of disease types such as cancer and inflammation. The complexity of carbohydrate structures and their derivatives makes their study a real challenge. Improving the isolation, separation, and characterization of carbohydrates and their glycoproteins is a subject of increasing scientific interest. With the development of new stationary phases and molecules that have affinity properties for glycoproteins, the isolation and separation of these compounds have advanced significantly. In addition to detection with mass spectrometry, the microarray platform has become an essential tool to characterize glycan structure and to study glycosylation-related biological interactions, by using probes as a means to interrogate the spotted or captured glycosylated molecules on the arrays. Furthermore, the high-throughput and reproducible nature of microarray platforms have been highlighted by its extensive applications in the field of biomarker validation, where a large number of samples must be analyzed multiple times. This review covers a brief survey of the other experimental methodologies that are currently being developed and used to study glycosylation and emphasizes methodologies that involve the use of microarray platforms. This review describes recent advances in several options of microarray platforms used in glycoprotein analysis, including glycoprotein arrays, glycan arrays, lectin arrays, and antibody/lectin arrays. The translational use of these arrays in applications related to characterization of cells and biomarker discovery is also included.
2010 Wiley Periodicals, Inc.
Figures




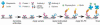



Similar articles
-
Analysis of serum protein glycosylation with antibody-lectin microarray for high-throughput biomarker screening.Methods Mol Biol. 2011;723:15-28. doi: 10.1007/978-1-61779-043-0_2. Methods Mol Biol. 2011. PMID: 21370056
-
Protein biomarkers in cancer: natural glycoprotein microarray approaches.Curr Opin Mol Ther. 2008 Dec;10(6):602-10. Curr Opin Mol Ther. 2008. PMID: 19051138 Free PMC article. Review.
-
Lectin microarrays for glycoprotein analysis.Methods Mol Biol. 2007;385:193-203. doi: 10.1007/978-1-59745-426-1_14. Methods Mol Biol. 2007. PMID: 18365713
-
Multiplexed antibody arrays for the discovery and validation of glycosylated protein biomarkers.Bioanalysis. 2009 Nov;1(8):1431-44. doi: 10.4155/bio.09.119. Bioanalysis. 2009. PMID: 21083093 Review.
-
Analysis of protein glycosylation and phosphorylation using liquid phase separation, protein microarray technology, and mass spectrometry.Methods Mol Biol. 2009;492:321-51. doi: 10.1007/978-1-59745-493-3_20. Methods Mol Biol. 2009. PMID: 19241043 Free PMC article.
Cited by
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2009-2010.Mass Spectrom Rev. 2015 May-Jun;34(3):268-422. doi: 10.1002/mas.21411. Epub 2014 May 26. Mass Spectrom Rev. 2015. PMID: 24863367 Free PMC article. Review.
-
Glycomic Approaches for the Discovery of Targets in Gastrointestinal Cancer.Front Oncol. 2016 Mar 9;6:55. doi: 10.3389/fonc.2016.00055. eCollection 2016. Front Oncol. 2016. PMID: 27014630 Free PMC article. Review.
-
A universal chemical enrichment method for mapping the yeast N-glycoproteome by mass spectrometry (MS).Mol Cell Proteomics. 2014 Jun;13(6):1563-72. doi: 10.1074/mcp.M113.036251. Epub 2014 Apr 1. Mol Cell Proteomics. 2014. PMID: 24692641 Free PMC article.
-
Analysis of glycan variation on glycoproteins from serum by the reverse lectin-based ELISA assay.J Proteome Res. 2014 Apr 4;13(4):2197-204. doi: 10.1021/pr401061c. Epub 2014 Mar 10. J Proteome Res. 2014. PMID: 24575722 Free PMC article.
-
David M. Lubman-The University of Michigan-A retrospective in research.Mass Spectrom Rev. 2023 Mar;42(2):643-651. doi: 10.1002/mas.21718. Epub 2021 Jul 21. Mass Spectrom Rev. 2023. PMID: 34289523 Free PMC article. Review. No abstract available.
References
-
- Adams EW, Ratner DM, Bokesch HR, McMahon JB, O'Keefe BR, Seeberger PH. Oligosaccharide and glycoprotein Microarrays as tools in HIV glycobiology: Glycan-dependent gp120/protein interactions. Chem Biol. 2004;6:875–881. - PubMed
-
- Amon S, Zamfir AD, Rizzi A. Glycosylation analysis of glycoproteins and proteoglycans using capillary electrophoresis-mass spectrometry strategies. Electrophoresis. 2008;29:2485–2507. - PubMed
-
- Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, Kochhar S, Sigrist H, Sprenger N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005;15:31–41. - PubMed
-
- Arnold JN, Saldova R, Hamid UMA, Rudd PM. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008;8:3284–3293. - PubMed
-
- Bertozzi CR, Sasisekharan R. Glycomics. In: Varki A, editor. Essentials of Glycobiology. Second Edition. The Consortium of Glycobiology Editors; California: 2009. chapter 48.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

