Palladium-catalyzed ligand-directed C-H functionalization reactions
- PMID: 20078038
- PMCID: PMC2836499
- DOI: 10.1021/cr900184e
Palladium-catalyzed ligand-directed C-H functionalization reactions
Figures



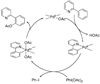
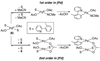
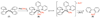











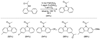

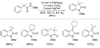

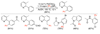
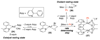
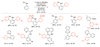









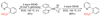
References
-
-
For pertinent reviews on C–H activation, see: Crabtree RH. Chem. Rev. 1985;85:245. Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc. Chem. Res. 1995;28:154. Shilov AE, Shul’pin GB. Chem. Rev. 1997;97:2879. Dyker G. Angew. Chem., Int. Ed. 1999;38:1698. Crabtree RH. J. Chem. Soc., Dalton Trans. 2001;2437 Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731. Labinger JA, Bercaw JE. Nature. 2002;417:507. Sezen B, Sames D. In: Handbook of C–H Transformations. Dyker G, editor. Weinheim, Germany: Wiley-VCH Velag GmbH & Co. KGaA; 2005. pp. 3–10. Yu JQ, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041. Godula K, Sames D. Science. 2006;312:67. Kalyani D, Sanford MS. In: Topics in Organometallic Chemistry: Directed Metalation. Chatani N, editor. Vol. 24. New York: Springer Berlin Heidelberg; 2007. pp. 85–116. Bergman RG. Nature. 2007;466:391. Hartwig JF. Nature. 2008;455:314.
-
-
- Cope AC, Siekman RW. J. Am. Chem. Soc. 1965;87:3272.
-
-
For examples, see: Davies HML, Beckwith REJ. Chem. Rev. 2003;103:2861. Daugulis O, Zaitsev VG, Shabashov D, Pham QN, Lazareva A. Synlett. 2006:3382. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174.
-
-
- Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

