Evaluation of mobilized peripheral blood CD34(+) cells from patients with severe coronary artery disease as a source of endothelial progenitor cells
- PMID: 20078384
- PMCID: PMC3919139
- DOI: 10.3109/14653240903493409
Evaluation of mobilized peripheral blood CD34(+) cells from patients with severe coronary artery disease as a source of endothelial progenitor cells
Abstract
Background aims: The distinction between hematopoietic stem cells (HSC) and endothelial progenitor cells (EPC) is poorly defined. Co-expression of CD34 antigen with vascular endothelial growth factor (VEGF) receptor (VEGFR2) is currently used to define EPC ( 1 ).
Methods: We evaluated the phenotypic and genomic characteristics of peripheral blood-derived CD34(+) cells in 22 granulocyte-colony-stimulating factor (G-CSF)-mobilized patients with severe coronary artery disease and assessed the influence of cell selection and storage on CD34(+) cell characteristics.
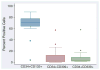
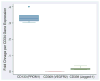
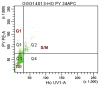
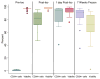
Results: The median CD34(+) cell contents in the products before and after enrichment with the Isolex 300i Magnetic Cell Selection System were 0.2% and 82.5%, respectively. Cell-cycle analysis showed that 80% of CD34(+) cells were in G0 stage; 70% of the isolated CD34(+) cells co-expressed CD133, a marker for more immature progenitors. However, less than 5% of the isolated CD34(+) cells co-expressed the notch receptor Jagged-1 (CD339) and only 2% of the isolated CD34(+) population were positive for VEGFR2 (CD309). Molecular assessment of the isolated CD34(+) cells demonstrated extremely low expression of VEGFR2 and endothelial nitric oxide synthase (eNOS) and high expression of VEGF-A. Overnight storage at 4 degrees C did not significantly affect CD34(+) cell counts and viability. Storage in liquid nitrogen for 7 weeks did not affect the percentage of CD34(+) cells but was associated with a 26% drop in cell viability.
Conclusions: We have demonstrated that the majority of isolated CD34(+) cells consist of immature and quiescent cells that lack prototypic markers of EPC. High VEGF-A gene expression might be one of the mechanisms for CD34(+) cell-induced angiogenesis.
Conflict of interest statement
Figures



Similar articles
-
Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors.Arterioscler Thromb Vasc Biol. 2007 Jul;27(7):1572-9. doi: 10.1161/ATVBAHA.107.144972. Epub 2007 May 10. Arterioscler Thromb Vasc Biol. 2007. PMID: 17495235
-
Endothelial precursor cells promote angiogenesis in hepatocellular carcinoma.World J Gastroenterol. 2012 Sep 21;18(35):4925-33. doi: 10.3748/wjg.v18.i35.4925. World J Gastroenterol. 2012. PMID: 23002366 Free PMC article.
-
Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/ Lin(-)CD45(-) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells.Exp Hematol. 2011 Apr;39(4):495-505. doi: 10.1016/j.exphem.2011.01.003. Epub 2011 Jan 14. Exp Hematol. 2011. PMID: 21238532
-
Biomarkers of anti-angiogenic therapy in metastatic colorectal cancer (mCRC): original data and review of the literature.Z Gastroenterol. 2011 Oct;49(10):1398-406. doi: 10.1055/s-0031-1281752. Epub 2011 Sep 30. Z Gastroenterol. 2011. PMID: 21964893 Review.
-
The involvement of endothelial progenitor cells in tumor angiogenesis.J Cell Mol Med. 2004 Jul-Sep;8(3):294-300. doi: 10.1111/j.1582-4934.2004.tb00319.x. J Cell Mol Med. 2004. PMID: 15491505 Free PMC article. Review.
Cited by
-
Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardial apoptosis in db/db mouse infarcted hearts.PLoS One. 2012;7(4):e35905. doi: 10.1371/journal.pone.0035905. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558265 Free PMC article.
-
Difference in Serum Endostatin Levels in Diabetic Patients with Critical Limb Ischemia Treated by Autologous Cell Therapy or Percutaneous Transluminal Angioplasty.Cell Transplant. 2018 Sep;27(9):1368-1374. doi: 10.1177/0963689718775628. Epub 2018 Jun 4. Cell Transplant. 2018. PMID: 29860903 Free PMC article.
-
Isolation of Foreign Material-Free Endothelial Progenitor Cells Using CD31 Aptamer and Therapeutic Application for Ischemic Injury.PLoS One. 2015 Jul 6;10(7):e0131785. doi: 10.1371/journal.pone.0131785. eCollection 2015. PLoS One. 2015. PMID: 26148001 Free PMC article.
-
Repertoire of endothelial progenitor cells mobilized by femoral artery ligation: a nonhuman primate study.J Cell Mol Med. 2012 Sep;16(9):2060-73. doi: 10.1111/j.1582-4934.2011.01501.x. J Cell Mol Med. 2012. PMID: 22128816 Free PMC article.
-
Circulating hematopoietic and endothelial progenitor cells in newborn infants: effects of gestational age, postnatal age and clinical stress in the first 3 weeks of life.Early Hum Dev. 2013 Jun;89(6):411-8. doi: 10.1016/j.earlhumdev.2012.12.006. Epub 2013 Jan 9. Early Hum Dev. 2013. PMID: 23312395 Free PMC article.
References
-
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. - PubMed
-
- Verfaillie CM, Pera MF, Lansdorp PM. Stem cells: hype and reality. Hematology (Am Soc Hematol Educ Program) 2002:369–91. - PubMed
-
- Zubair AC, Silberstein L, Ritz J. Adult hematopoietic stem cell plasticity. Transfusion. 2002;42:1096–101. - PubMed
-
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

