Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation
- PMID: 20078491
- PMCID: PMC4565618
- DOI: 10.1111/j.1360-0443.2009.02780.x
Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation
Abstract
Aims: To examine the effects of reduced nicotine cigarettes on smoking behavior, toxicant exposure, dependence and abstinence.
Design: Randomized, parallel arm, semi-blinded study. Setting University of Minnesota Tobacco Use Research Center.
Interventions: Six weeks of: (i) 0.05 mg nicotine yield cigarettes; (ii) 0.3 mg nicotine yield cigarettes; or (iii) 4 mg nicotine lozenge; 6 weeks of follow-up. Measurements Compensatory smoking behavior, biomarkers of exposure, tobacco dependence, tobacco withdrawal and abstinence rate.
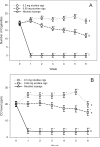
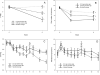
Findings: Unlike the 0.3 mg cigarettes, 0.05 mg cigarettes were not associated with compensatory smoking behaviors. Furthermore, the 0.05 mg cigarettes and nicotine lozenge were associated with reduced carcinogen exposure, nicotine dependence and product withdrawal scores. The 0.05 mg cigarette was associated with greater relief of withdrawal from usual brand cigarettes than the nicotine lozenge. The 0.05 mg cigarette led to a significantly higher rate of cessation than the 0.3 mg cigarette and a similar rate as nicotine lozenge.
Conclusion: The 0.05 mg nicotine yield cigarettes may be a tobacco product that can facilitate cessation; however, future research is clearly needed to support these preliminary findings.
Trial registration: ClinicalTrials.gov NCT00777569.
Figures
Comment in
-
Commentary on Hatsukami et al. (2010): building the science base for tobacco product regulation-nicotine reduction.Addiction. 2010 Feb;105(2):356-7. doi: 10.1111/j.1360-0443.2009.02882.x. Addiction. 2010. PMID: 20078492 No abstract available.
References
-
- Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Institute of Medicine, National Academy Press; Washington, DC: 2001. - PubMed
-
- Royal College of Physicians . A Report by the Tobacco Advisory Group of the Royal College of Physicians. Royal College of Physicians; London: 2007. Harm reduction in nicotine addiction. Helping people who can't quit. p. 176.
-
- Hatsukami D, Hecht SS. Hope or hazard: what research tells us about ‘potentially reduced-exposure’ tobacco products. University of Minnesota Transdisciplinary Tobacco Use Research Center; Minneapolis: 2005. [9 December 2009]. Available from http://www.rwjf.org/files/research/Hope%20or%20Hazard.pdf.
-
- US Department of Health and Human Services (USDHHS) The Health Consequences of Smoking: Nicotine and Addiction. A Report of the Surgeon General. USDHHS; Rockville, MD: 1988.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

