Inosine monophosphate dehydrogenase variability in renal transplant patients on long-term mycophenolate mofetil therapy
- PMID: 20078611
- PMCID: PMC2830596
- DOI: 10.1111/j.1365-2125.2009.03542.x
Inosine monophosphate dehydrogenase variability in renal transplant patients on long-term mycophenolate mofetil therapy
Abstract
What is already known about this subject: * Mycophenolic acid (MPA) is a potent, selective and reversible inhibitor of inosine 5'-monophosphate dehydrogenase (IMPDH), the rate-limiting enzyme for de novo guanosine triphosphate biosynthesis. * The large IMPDH interindividual variability could be responsible for the differences in therapeutic effects and side-effects observed with MPA. * Induction of IMPDH activity has been observed in whole blood during immunosuppressive therapy.
What this study adds: * Our data were acquired in long-term mycophenolate mofetil-treated renal transplant recipients on different combinations of immunosuppressive agents (ciclosporin, tacrolimus, sirolimus) and with different treatment duration (up to 8.8 years post transplant). * The increasing trend in IMPDH activity that we observed throughout our 12-month observation period was significantly higher in rejecting than in nonrejecting subjects.
Aims: Long-term mycophenolate mofetil (MMF) therapy may induce inosine 5'-monophosphate dehydrogenase (IMPDH) activity in peripheral blood mononuclear cells (PBMCs), thus decreasing MMF immunosuppressive properties. Pharmacodynamic monitoring was used to investigate whether biological activity is altered after long-term therapy.
Methods: IMPDH activity was measured in PBMC samples from 54 stable kidney transplant patients, already on MMF (for at least 3 months), before (t(0)) and 2 h after (t(2)) MMF morning dose administration; levels were monitored for up to 15 months, together with total mycophenolic acid (MPA) and free MPA concentrations.
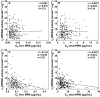
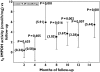
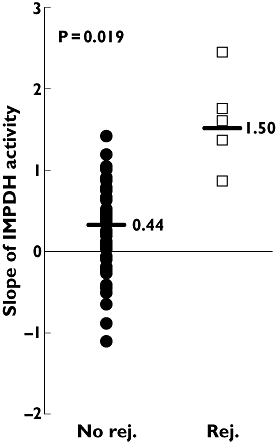
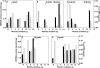
Results: During the 15 months' monitoring, t(0) IMPDH activity in transplant recipients increased from 5.9 +/- 3.7 nmol h(-1) mg(-1)[95% confidence interval (CI) 4.9, 6.9] to 9.0 +/- 3.9 nmol h(-1) mg(-1) (95% CI 7.2, 10.8), with an intra- and interpatient variability of 28% and 42%. Five patients experienced acute rejection during the follow-up: t(0) IMPDH activity was increased during rejection vs. nonrejection, and the trend was significantly higher in rejecting than in nonrejecting subjects for the whole monitoring period.
Conclusions: Even though a correlation has been found between IMPDH activity and rejection, its efficacy as a predictive tool in long-term transplant outcomes may be affected by high interpatient variability; on the other hand, continuous monitoring of the IMPDH trend could make an effective prognostic parameter of rejection. Other trials also including pre-transplant data on both IMPDH expression and activity are warranted to better assess their role as biomarkers for MPA effect in clinical practice.
Figures

Similar articles
-
Effect of mycophenolate mofetil on IMP dehydrogenase after the first dose and after long-term treatment in renal transplant recipients.Int J Clin Pharmacol Ther. 2003 Oct;41(10):470-6. doi: 10.5414/cpp41470. Int J Clin Pharmacol Ther. 2003. PMID: 14703953 Clinical Trial.
-
Effect of mycophenolic acid on inosine monophosphate dehydrogenase (IMPDH) activity in liver transplant patients.Clin Res Hepatol Gastroenterol. 2020 Sep;44(4):543-550. doi: 10.1016/j.clinre.2019.12.001. Epub 2020 Jan 7. Clin Res Hepatol Gastroenterol. 2020. PMID: 31924555
-
Inosine monophosphate dehydrogenase activity depends on plasma concentrations of mycophenolic acid and its glucuronides in kidney transplant recipients.Clin Chim Acta. 2009 Nov;409(1-2):56-61. doi: 10.1016/j.cca.2009.08.016. Epub 2009 Aug 31. Clin Chim Acta. 2009. PMID: 19723513
-
Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update.Arch Toxicol. 2014 Jul;88(7):1351-89. doi: 10.1007/s00204-014-1247-1. Epub 2014 May 4. Arch Toxicol. 2014. PMID: 24792322 Review.
-
Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients.Clin Pharmacokinet. 2007;46(1):13-58. doi: 10.2165/00003088-200746010-00002. Clin Pharmacokinet. 2007. PMID: 17201457 Review.
Cited by
-
Inosine monophosphate dehydrogenase activity in paediatrics: age-related regulation and response to mycophenolic acid.Eur J Clin Pharmacol. 2012 Jun;68(6):913-22. doi: 10.1007/s00228-011-1203-4. Epub 2012 Jan 25. Eur J Clin Pharmacol. 2012. PMID: 22274404
-
Pharmacodynamic assessment of mycophenolic acid in resting and activated target cell population during the first year after renal transplantation.Br J Clin Pharmacol. 2020 Jun;86(6):1100-1112. doi: 10.1111/bcp.14218. Epub 2020 Feb 16. Br J Clin Pharmacol. 2020. PMID: 31925806 Free PMC article.
-
Treatment of severe lupus nephritis: the new horizon.Nat Rev Nephrol. 2015 Jan;11(1):46-61. doi: 10.1038/nrneph.2014.215. Epub 2014 Nov 25. Nat Rev Nephrol. 2015. PMID: 25421826 Review.
-
Expression of IMPDH mRNA after mycophenolate administration in male volunteers.Biomed Res Int. 2014;2014:870209. doi: 10.1155/2014/870209. Epub 2014 Jul 1. Biomed Res Int. 2014. PMID: 25105143 Free PMC article. Clinical Trial.
-
Immune Monitoring of Mycophenolate Mofetil Activity in Healthy Volunteers Using Ex Vivo T Cell Function Assays.Pharmaceutics. 2023 May 31;15(6):1635. doi: 10.3390/pharmaceutics15061635. Pharmaceutics. 2023. PMID: 37376083 Free PMC article.
References
-
- Bullingham RE, Nicholls A, Hale M. Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant Proc. 1996;28:925–9. - PubMed
-
- Natsumeda Y, Ohno S, Kawasaki H, Konno Y, Weber G, Suzuki K. Two distinct cDNAs for human IMP dehydrogenase. J Biol Chem. 1990;265:5292–5. - PubMed
-
- Collart FR, Huberman E. Cloning and sequence analysis of the human and Chinese hamster inosine-5′-monophosphate dehydrogenase cDNAs. J Biol Chem. 1988;263:15769–72. - PubMed
-
- Gu JJ, Spychala J, Mitchell BS. Regulation of the human inosine monophosphate dehydrogenase type I gene. Utilization of alternative promoters. J Biol Chem. 1997;272:4458–66. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

