Assessment of the pharmacokinetics of co-administered maraviroc and raltegravir
- PMID: 20078612
- PMCID: PMC2830597
- DOI: 10.1111/j.1365-2125.2009.03546.x
Assessment of the pharmacokinetics of co-administered maraviroc and raltegravir
Abstract
What is already known about this subject: * Maraviroc is a CCR5 receptor antagonist, while raltegravir is a HIV-1 integrase inhibitor. * Based on the known metabolic pathways (CYP3A4 for maraviroc and UGT1A1 for raltegravir), interaction between the two drugs is unlikely. However, unexpected interactions have been reported for other antiretroviral drugs. * As both these drugs are likely to be used in combination, this study evaluated the pharmacokinetic interaction between them.
What this study adds: * Relative to individual monotherapy, co-administration resulted in a 20% and 33% decrease in mean C(max), and 14% and 37% decrease in mean AUC of maraviroc and raltegravir, respectively. * Co-administration was generally safe and well tolerated in healthy subjects. * These changes are not likely to be clinically relevant, thus no dose adjustment is necessary.
Aims: To assess the two-way pharmacokinetic interaction between maraviroc and raltegravir.
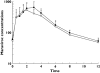
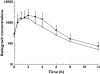
Methods: In this open-label, multiple-dose, fixed-sequence study, 18 healthy, human immunodeficiency virus (HIV)-seronegative subjects received the following: days 1-3 raltegravir 400 mg q12h, days 4-5 washout, days 6-11 maraviroc 300 mg q12h, and days 12-14 raltegravir 400 mg q12h + maraviroc 300 mg q12h. Serial 12-h blood samples were collected on days 3 (raltegravir), 11 (maraviroc) and 14 (raltegravir + maraviroc). Plasma samples were assayed by validated liquid chromatography tandem mass spectrometry assays. Test/reference ratios and 95% confidence intervals (CIs) were determined for pharmacokinetic parameters.
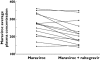
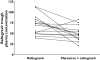
Results: For maraviroc, the test/reference % ratio (95% CI) for AUC(tau) was 85.8 (78.7, 93.5), for C(max) was 79.5 (64.8, 97.5) and for C(min) was 90.3 (84.2, 96.9). For raltegravir, the test/reference % ratio (95% CI) for AUC(tau) was 63.3 (41.0, 97.6), for C(max) was 66.8 (37.1, 120.0) and for C(min) was 72.4 (55.1, 95.2). In all subjects, maraviroc average concentrations (AUC(tau) divided by 12) were >100 ng ml(-1), the threshold value below which there is an increased risk of virological failure. Based on clinical experience for raltegravir, mean C(min) decreases >60% are considered to be clinically relevant for short-term activity; however, in the present study mean changes were only 28% and thus not considered to be of clinical relevance.
Conclusions: Co-administration of maraviroc and raltegravir decreased systemic exposure of both drugs; however, these are not likely to be clinically relevant. Safety and efficacy studies may help in understanding the role of this combination in the treatment of HIV infection.
Figures
References
-
- World Heath Organization. 2007 AIDS Epidemic Update. Available at http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf (last accessed 2 October 2009.
-
- Wilson LE, Gallant JE. HIV/AIDS: the management of treatment-experienced HIV-infected patients: new drugs and drug combinations. Clin Infect Dis. 2009;48:214–21. - PubMed
-
- Marviroc (Selzentry®). United States Prescribing Information. New York: Pfizer Inc.; 2008.
-
- MacArthur RD, Novak RM. Reviews of anti-infective agents: Maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47:236–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

