Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and FOLFOX response in colorectal cancer patients
- PMID: 20078613
- PMCID: PMC2830598
- DOI: 10.1111/j.1365-2125.2009.03556.x
Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and FOLFOX response in colorectal cancer patients
Abstract
What is already known about this subject: * Numerous clinical studies, including a few prospective ones, have reported conflicting results on the impact of gene polymorphisms related to fluorouracil (FU) and oxaliplatin pharmacodynamics.
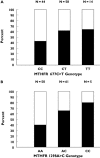
What this study adds: * This prospective study is the first to report that clinical response to FOLFOX is significantly related to methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms (677C-->T and 1298A-->C), with a response rate of 37, 53, 63 and 80% in patients harbouring no, one, two or three favourable MTHFR alleles, respectively. * Only polymorphisms of genes related to oxaliplatin pharmacodynamics (GSTpi 105Ile-->Val and XPD 751Ly-->Gln) influenced progression-free survival. * These results corroborate the observation that response was related to the cumulative FU dose, whereas progression-free survival was related to the cumulative oxaliplatin dose.
Aims: To test prospectively the predictive value of germinal gene polymorphisms related to fluorouracil (FU) and oxaliplatin (Oxa) pharmacodynamics on toxicity and responsiveness of colorectal cancer (CRC) patients receiving FOLFOX therapy.
Methods: Advanced CRC patients (n= 117) receiving FOLFOX 7 therapy were enrolled. Gene polymorphisms relevant for FU [thymidylate synthase (TYMS, 28 bp repeats including the G-->C mutation + 6 bp deletion in 3'UTR), methylenetetrahydrofolate reductase (MTHFR, 677C-->T, 1298A-->C), dihydropyrimidine deshydrogenase (IVS14+1G-->A) and Oxa: glutathione S-transferase (GST) pi (105Ile-->Val, 114Ala-->Val), excision repair cross-complementing group 1 (ERCC1) (118AAT-->AAC), ERCC2 (XPD, 751Lys-->Gln) and XRCC1 (399Arg-->Gln)] were determined (blood mononuclear cells).
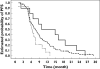
Results: None of the genotypes was predictive of toxicity. Response rate (54.7% complete response + partial response) was related to FU pharmacogenetics, with both 677C-->T (P= 0.042) and 1298A-->C (P= 0.004) MTHFR genotypes linked to clinical response. Importantly, the score of favourable MTHFR alleles (677T and 1298C) was positively linked to response, with response rates of 37.1, 53.3, 62.5 and 80.0% in patients bearing no, one, two or three favourable alleles, respectively (P= 0.040). Polymorphisms of genes related to Oxa pharmacodynamics showed an influence on progression-free survival, with a better outcome in patients bearing GSTpi 105 Val/Val genotype or XPD 751Lys-containing genotype (P= 0.054).
Conclusions: These results show that response to FOLFOX therapy in CRC patients may be driven by MTHFR germinal polymorphisms.
Figures
 ); SD+PD (
); SD+PD ( )
)
 ); Score 1 (
); Score 1 ( ); Score 2 (—)
); Score 2 (—)Similar articles
-
Which gene is a dominant predictor of response during FOLFOX chemotherapy for the treatment of metastatic colorectal cancer, the MTHFR or XRCC1 gene?Ann Surg Oncol. 2006 Nov;13(11):1379-85. doi: 10.1245/s10434-006-9112-y. Epub 2006 Sep 29. Ann Surg Oncol. 2006. PMID: 17009149
-
Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial.J Clin Oncol. 2009 Nov 20;27(33):5519-28. doi: 10.1200/JCO.2008.21.6283. Epub 2009 Oct 26. J Clin Oncol. 2009. PMID: 19858398 Clinical Trial.
-
The Impact of Thymidylate Synthase and Methylenetetrahydrofolate Reductase Genotypes on Sensitivity to 5-Fluorouracil Treatment in Colorectal Cancer Cells.Acta Med Iran. 2017 Dec;55(12):751-758. Acta Med Iran. 2017. PMID: 29373881
-
Methylenetetrahydrofolate reductase (MTHFR) variants and fluorouracil-based treatments in colorectal cancer.Pharmacogenomics. 2007 Nov;8(11):1561-6. doi: 10.2217/14622416.8.11.1561. Pharmacogenomics. 2007. PMID: 18034621 Review.
-
The evolving role of oxaliplatin in the management of colorectal cancer.Colorectal Dis. 2003 Nov;5 Suppl 3:10-9. doi: 10.1046/j.1463-1318.5.s3.3.x. Colorectal Dis. 2003. PMID: 23573556 Review.
Cited by
-
Prediction of exposure-driven myelotoxicity of continuous infusion 5-fluorouracil by a semi-physiological pharmacokinetic-pharmacodynamic model in gastrointestinal cancer patients.Cancer Chemother Pharmacol. 2020 Apr;85(4):711-722. doi: 10.1007/s00280-019-04028-5. Epub 2020 Mar 9. Cancer Chemother Pharmacol. 2020. PMID: 32152679 Free PMC article.
-
ERCC1 and XPD/ERCC2 polymorphisms' predictive value of oxaliplatin-based chemotherapies in advanced colorectal cancer has an ethnic discrepancy: a meta-analysis.J Clin Lab Anal. 2012 Jan;26(1):10-5. doi: 10.1002/jcla.20494. J Clin Lab Anal. 2012. PMID: 24833529 Free PMC article.
-
The Road so Far in Colorectal Cancer Pharmacogenomics: Are We Closer to Individualised Treatment?J Pers Med. 2020 Nov 19;10(4):237. doi: 10.3390/jpm10040237. J Pers Med. 2020. PMID: 33228198 Free PMC article. Review.
-
Pathway analysis of genetic variants in folate-mediated one-carbon metabolism-related genes and survival in a prospectively followed cohort of colorectal cancer patients.Cancer Med. 2018 Jul;7(7):2797-2807. doi: 10.1002/cam4.1407. Epub 2018 May 29. Cancer Med. 2018. PMID: 29845757 Free PMC article.
-
Integrating TYMS, KRAS and BRAF testing in patients with metastatic colorectal cancer.World J Gastroenterol. 2017 Aug 28;23(32):5913-5924. doi: 10.3748/wjg.v23.i32.5913. World J Gastroenterol. 2017. PMID: 28932083 Free PMC article.
References
-
- De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, De Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. - PubMed
-
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–7. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. - PubMed
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. - PubMed
-
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, De Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

