Expression and regulation of Sclerotinia sclerotiorum necrosis and ethylene-inducing peptides (NEPs)
- PMID: 20078775
- PMCID: PMC6640525
- DOI: 10.1111/j.1364-3703.2009.00571.x
Expression and regulation of Sclerotinia sclerotiorum necrosis and ethylene-inducing peptides (NEPs)
Abstract
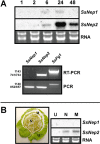
Successful host colonization by necrotrophic plant pathogens requires the induction of plant cell death to provide the nutrients needed for infection establishment and progression. We have cloned two genes encoding necrosis and ethylene-inducing peptides from Sclerotinia sclerotiorum, which we named SsNep1 and SsNep2. The peptides encoded by these genes induce necrosis when expressed transiently in tobacco leaves. SsNep1 is expressed at a very low level relative to SsNep2 during infection. The expression of SsNep2 was induced by contact with solid surfaces and occurred in both the necrotic zone and at the leading margin of the infection. SsNep2 expression was dependent on calcium and cyclic adenosine monophosphate signalling, as compounds affecting these pathways reduced or abolished SsNep2 expression coincident with a partial or total loss of virulence.
Figures






Similar articles
-
SsNEP2 Contributes to the Virulence of Sclerotinia sclerotiorum.Pathogens. 2022 Apr 7;11(4):446. doi: 10.3390/pathogens11040446. Pathogens. 2022. PMID: 35456121 Free PMC article.
-
SsNEP2 Plays a Role in the Interaction Between Sclerotinia sclerotiorum and Coniothyrium minitans.J Fungi (Basel). 2025 Feb 16;11(2):151. doi: 10.3390/jof11020151. J Fungi (Basel). 2025. PMID: 39997445 Free PMC article.
-
Factors governing the regulation of Sclerotinia sclerotiorum cutinase A and polygalacturonase 1 during different stages of infection.Can J Microbiol. 2012 May;58(5):605-16. doi: 10.1139/w2012-031. Epub 2012 Apr 23. Can J Microbiol. 2012. PMID: 22524557
-
Sclerotinia sclerotiorum: when "to be or not to be" a pathogen?FEMS Microbiol Lett. 2005 Oct 15;251(2):177-84. doi: 10.1016/j.femsle.2005.07.040. FEMS Microbiol Lett. 2005. PMID: 16112822 Review.
-
Mechanisms of Broad Host Range Necrotrophic Pathogenesis in Sclerotinia sclerotiorum.Phytopathology. 2018 Oct;108(10):1128-1140. doi: 10.1094/PHYTO-06-18-0197-RVW. Epub 2018 Aug 30. Phytopathology. 2018. PMID: 30048598 Review.
Cited by
-
Identification and Functional Analysis of NLP-Encoding Genes from the Postharvest Pathogen Penicillium expansum.Microorganisms. 2019 Jun 15;7(6):175. doi: 10.3390/microorganisms7060175. Microorganisms. 2019. PMID: 31208074 Free PMC article.
-
Fungal effectors versus defense-related genes of B. juncea and the status of resistant transgenics against fungal pathogens.Front Plant Sci. 2023 Jun 8;14:1139009. doi: 10.3389/fpls.2023.1139009. eCollection 2023. Front Plant Sci. 2023. PMID: 37360735 Free PMC article. Review.
-
A glycosylphosphatidylinositol-anchored protein from Alternaria alternata triggers cell death and negatively modulates immunity responses in chrysanthemum.Plant Cell Rep. 2024 Nov 18;43(12):283. doi: 10.1007/s00299-024-03372-y. Plant Cell Rep. 2024. PMID: 39557715
-
Genetic breakthroughs in the Brassica napus-Sclerotinia sclerotiorum interactions.Front Plant Sci. 2023 Nov 23;14:1276055. doi: 10.3389/fpls.2023.1276055. eCollection 2023. Front Plant Sci. 2023. PMID: 38078117 Free PMC article. Review.
-
Association mapping in sunflower for Sclerotinia Head Rot resistance.BMC Plant Biol. 2012 Jun 18;12:93. doi: 10.1186/1471-2229-12-93. BMC Plant Biol. 2012. PMID: 22708963 Free PMC article.
References
-
- Amsellem, Z. , Cohen, B.A. and Gressel, J. (2002) Engineering hypovirulence in a mycoherbicidal fungus for efficient weed control. Nat. Biotechnol. 20, 1035–1039. - PubMed
-
- Bailey, B.A. (1995) Purification of a protein from culture filtrate of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca . Phytopathology, 85, 1250–1255.
-
- Boland, G.J. and Hall, R. (1994) Index of plant hosts of Sclerotinia sclerotiorum . Can. J. Plant Pathol. 16, 93–100.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

