Vpu serine 52 dependent counteraction of tetherin is required for HIV-1 replication in macrophages, but not in ex vivo human lymphoid tissue
- PMID: 20078884
- PMCID: PMC2823648
- DOI: 10.1186/1742-4690-7-1
Vpu serine 52 dependent counteraction of tetherin is required for HIV-1 replication in macrophages, but not in ex vivo human lymphoid tissue
Abstract
Background: The human immunodeficiency virus type 1 (HIV-1) Vpu protein degrades CD4 and counteracts a restriction factor termed tetherin (CD317; Bst-2) to enhance virion release. It has been suggested that both functions can be genetically separated by mutation of a serine residue at position 52. However, recent data suggest that the S52 phosphorylation site is also important for the ability of Vpu to counteract tetherin. To clarify this issue, we performed a comprehensive analysis of HIV-1 with a mutated casein kinase-II phosphorylation site in Vpu in various cell lines, primary blood lymphocytes (PBL), monocyte-derived macrophages (MDM) and ex vivo human lymphoid tissue (HLT).
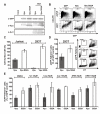
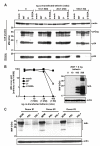
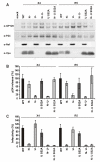
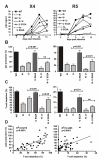
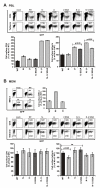
Results: We show that mutation of serine 52 to alanine (S52A) entirely disrupts Vpu-mediated degradation of CD4 and strongly impairs its ability to antagonize tetherin. Furthermore, casein-kinase II inhibitors blocked the ability of Vpu to degrade tetherin. Overall, Vpu S52A could only overcome low levels of tetherin, and its activity decreased in a manner dependent on the amount of transiently or endogenously expressed tetherin. As a consequence, the S52A Vpu mutant virus was unable to replicate in macrophages, which express high levels of this restriction factor. In contrast, HIV-1 Vpu S52A caused CD4+ T-cell depletion and spread efficiently in ex vivo human lymphoid tissue and PBL, most likely because these cells express comparably low levels of tetherin.
Conclusion: Our data explain why the effect of the S52A mutation in Vpu on virus release is cell-type dependent and suggest that a reduced ability of Vpu to counteract tetherin impairs HIV-1 replication in macrophages, but not in tissue CD4+ T cells.
Figures
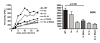
Similar articles
-
Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism.J Virol. 2009 Aug;83(16):7931-47. doi: 10.1128/JVI.00242-09. Epub 2009 Jun 10. J Virol. 2009. PMID: 19515779 Free PMC article.
-
The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu.Retrovirology. 2009 Sep 8;6:80. doi: 10.1186/1742-4690-6-80. Retrovirology. 2009. PMID: 19737401 Free PMC article.
-
Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu.J Virol. 2010 Apr;84(8):4089-94. doi: 10.1128/JVI.01549-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147395 Free PMC article.
-
HIV-1 Vpu - an ion channel in search of a job.Biochim Biophys Acta. 2014 Apr;1838(4):1074-81. doi: 10.1016/j.bbamem.2013.06.029. Epub 2013 Jul 3. Biochim Biophys Acta. 2014. PMID: 23831603 Free PMC article. Review.
-
β-TrCP dependency of HIV-1 Vpu-induced downregulation of CD4 and BST-2/tetherin.Curr HIV Res. 2012 Jun;10(4):307-14. doi: 10.2174/157016212800792441. Curr HIV Res. 2012. PMID: 22524179 Review.
Cited by
-
HIV replication and latency in monocytes and macrophages.Semin Immunol. 2021 Jan;51:101472. doi: 10.1016/j.smim.2021.101472. Epub 2021 Feb 27. Semin Immunol. 2021. PMID: 33648815 Free PMC article. Review.
-
HIV-1 Nef in macrophage-mediated disease pathogenesis.Int Rev Immunol. 2012 Dec;31(6):432-50. doi: 10.3109/08830185.2012.737073. Int Rev Immunol. 2012. PMID: 23215766 Free PMC article. Review.
-
Tetherin/BST-2 is essential for the formation of the intracellular virus-containing compartment in HIV-infected macrophages.Cell Host Microbe. 2012 Sep 13;12(3):360-72. doi: 10.1016/j.chom.2012.07.011. Cell Host Microbe. 2012. PMID: 22980332 Free PMC article.
-
Antiviral inhibition of enveloped virus release by tetherin/BST-2: action and counteraction.Viruses. 2011 May;3(5):520-40. doi: 10.3390/v3050520. Epub 2011 May 6. Viruses. 2011. PMID: 21994744 Free PMC article. Review.
-
Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef.Retrovirology. 2011 Apr 19;8:26. doi: 10.1186/1742-4690-8-26. Retrovirology. 2011. PMID: 21504576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

