Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis
- PMID: 20079333
- PMCID: PMC2885975
- DOI: 10.1016/j.cell.2009.11.027
Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis
Abstract
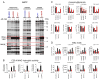
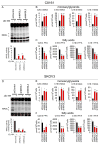
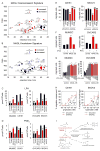
Tumor cells display progressive changes in metabolism that correlate with malignancy, including development of a lipogenic phenotype. How stored fats are liberated and remodeled to support cancer pathogenesis, however, remains unknown. Here, we show that the enzyme monoacylglycerol lipase (MAGL) is highly expressed in aggressive human cancer cells and primary tumors, where it regulates a fatty acid network enriched in oncogenic signaling lipids that promotes migration, invasion, survival, and in vivo tumor growth. Overexpression of MAGL in nonaggressive cancer cells recapitulates this fatty acid network and increases their pathogenicity-phenotypes that are reversed by an MAGL inhibitor. Impairments in MAGL-dependent tumor growth are rescued by a high-fat diet, indicating that exogenous sources of fatty acids can contribute to malignancy in cancers lacking MAGL activity. Together, these findings reveal how cancer cells can co-opt a lipolytic enzyme to translate their lipogenic state into an array of protumorigenic signals. PAPERFLICK:
Figures


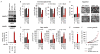

Comment in
-
Chewing the fat on tumor cell metabolism.Cell. 2010 Jan 8;140(1):28-30. doi: 10.1016/j.cell.2009.12.037. Cell. 2010. PMID: 20085702
References
-
- Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. - PubMed
-
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

