Intrinsic signal optical imaging of brain function using short stimulus delivery intervals
- PMID: 20079373
- PMCID: PMC2832718
- DOI: 10.1016/j.jneumeth.2010.01.009
Intrinsic signal optical imaging of brain function using short stimulus delivery intervals
Abstract
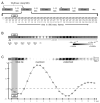
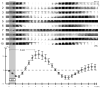
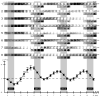
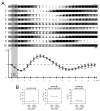
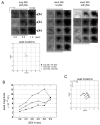
Intrinsic signal optical imaging (ISOI) can be used to map cortical function and organization. Because its detected signal lasts 10+s consisting of three phases, trials are typically collected using a long (tens of seconds) stimulus delivery interval (SDI) at the expense of efficiency, even when interested in mapping only the first signal phase (e.g., ISOI initial dip). It is unclear how the activity profile can change when stimuli are delivered at shorter intervals, and whether a short SDI can be implemented to improve efficiency. The goals of the present study are twofold: characterize the ISOI activity profile when multiple stimuli are delivered at 4s intervals, and determine whether successful mapping can be attained from trials collected using an SDI of 4s (offering >10x increase in efficiency). Our results indicate that four stimuli delivered 4s apart evoke an activity profile different from the triphasic signal, consisting of signal dips in a series at the same frequency as the stimuli despite a strong rise in signal prior to the 2nd to 4th stimuli. Visualization of such signal dips is dependent on using a baseline immediately prior to every stimulus. Use of the 4-s SDI is confirmed to successfully map activity with a similar location in peak activity and increased areal extent and peak magnitude compared to using a long SDI. Additional experiments were performed to begin addressing issues such as SDI temporal jittering, response magnitude as a function of SDI duration, and application for successful mapping of cortical function topography.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures

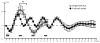

Similar articles
-
The triphasic intrinsic signal: implications for functional imaging.J Neurosci. 2007 Apr 25;27(17):4572-86. doi: 10.1523/JNEUROSCI.0326-07.2007. J Neurosci. 2007. PMID: 17460070 Free PMC article.
-
Varying the degree of single-whisker stimulation differentially affects phases of intrinsic signals in rat barrel cortex.J Neurophysiol. 1999 Feb;81(2):692-701. doi: 10.1152/jn.1999.81.2.692. J Neurophysiol. 1999. PMID: 10036270
-
Mapping functional activity in rodent cortex using optical intrinsic signals.Cereb Cortex. 1994 Mar-Apr;4(2):195-204. doi: 10.1093/cercor/4.2.195. Cereb Cortex. 1994. PMID: 8038568
-
Visualizing Adult Cortical Plasticity Using Intrinsic Signal Optical Imaging.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 9. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 9. PMID: 26844321 Free Books & Documents. Review.
-
Quantitative In Vivo Imaging of Tissue Absorption, Scattering, and Hemoglobin Concentration in Rat Cortex Using Spatially Modulated Structured Light.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 12. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 12. PMID: 26844326 Free Books & Documents. Review.
Cited by
-
Optical imaging reveals functional domains in primate sensorimotor cortex.Neuroimage. 2020 Nov 1;221:117188. doi: 10.1016/j.neuroimage.2020.117188. Epub 2020 Jul 23. Neuroimage. 2020. PMID: 32711067 Free PMC article.
-
Astrocyte-neuron lactate shuttle plays a pivotal role in sensory-based neuroprotection in a rat model of permanent middle cerebral artery occlusion.Sci Rep. 2023 Aug 7;13(1):12799. doi: 10.1038/s41598-023-39574-9. Sci Rep. 2023. PMID: 37550353 Free PMC article.
-
Appearance of fast astrocytic component in voltage-sensitive dye imaging of neural activity.Mol Brain. 2015 Jun 5;8:35. doi: 10.1186/s13041-015-0127-9. Mol Brain. 2015. PMID: 26043770 Free PMC article.
-
Existence of Initial Dip for BCI: An Illusion or Reality.Front Neurorobot. 2018 Oct 26;12:69. doi: 10.3389/fnbot.2018.00069. eCollection 2018. Front Neurorobot. 2018. PMID: 30416440 Free PMC article. Review.
-
Astrocyte-neuron lactate shuttle plays a pivotal role in sensory-based neuroprotection in a rat model of permanent middle cerebral artery occlusion.Res Sq [Preprint]. 2023 Mar 30:rs.3.rs-2698138. doi: 10.21203/rs.3.rs-2698138/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Aug 7;13(1):12799. doi: 10.1038/s41598-023-39574-9. PMID: 37034797 Free PMC article. Updated. Preprint.
References
-
- Bandettini PA, Cox RW. Event-related fMRI contrast when using constant interstimulus interval: theory and experiment. Magn Reson Med. 2000;43:540–548. - PubMed
-
- Chen-Bee CH, Kwon MC, Masino SA, Frostig RD. Areal extent quantification of functional representations using intrinsic signal optical imaging. J Neurosci Methods. 1996;68:27–37. - PubMed
-
- Chen-Bee CH, Polley DB, Brett-Green B, Prakash N, Kwon MC, Frostig RD. Visualizing and quantifying evoked cortical activity assessed with intrinsic signal imaging. J Neurosci Methods. 2000;97:157–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

