Expression of zebrafish (Danio rerio) monoamine oxidase (MAO) in Pichia pastoris: purification and comparison with human MAO A and MAO B
- PMID: 20079438
- PMCID: PMC2829425
- DOI: 10.1016/j.pep.2010.01.005
Expression of zebrafish (Danio rerio) monoamine oxidase (MAO) in Pichia pastoris: purification and comparison with human MAO A and MAO B
Abstract
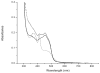
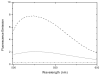
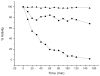
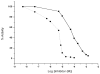
The expression, purification and characterization of zebrafish monoamine oxidase (zMAO) using the methylotropic yeast Pichia pastoris expression system is described. A 1L fermentation culture of Pichia pastoris containing the gene encoding zMAO under control of the methanol oxidase promotor expresses approximately 200mg of zMAO exhibiting 300 U of total activity. The enzyme is found in the mitochondrial fraction of the expression host and is purified in a 30% yield as a homogenous species with a M(r) of approximately 60,000 on SDS-PAGE and a mass of 58,525+/-40 Da from MALDI-TOF measurements. The zMAO preparation contains one mole of covalent flavin cofactor per mole of enzyme and exhibits >80% functionality. The covalent flavin exhibits fluorescence and EPR spectral properties consistent with known properties of 8 alpha-S-cysteinyl FAD. Chemical degradation of the flavin peptide results in the liberation of FAD. zMAO exhibits no immuno-chemical cross-reactivity with polyclonal anti-sera raised against human MAO A. The enzyme preparation exhibits reasonable thermostability up to a temperature of 30 degrees C. Benzylamine is oxidized with a k(cat) value of 4.7+/-0.1 min(-1) (K(m)=82+/-9 microM) and the enzyme oxidizes phenylethylamine with a k(cat) value of 204 min(-1) (K(m)=86+/-13 microM). The K(m) (O(2)) values determined for zMAO using either benzylamine or phenylethylamine as substrates ranges from 108(+/-5) to 140(+/-21)microM. The functional behavior of this teleost MAO relative to human MAO A and MAO B is discussed.
(c) 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Kumar MJ, Nicholls DG, Andersen JK. Oxidative alpha-ketoglutarate dehydrogenase inhibition via subtle elevations in monoamine oxidase B levels results in loss of spare respiratory capacity - Implications for Parkinson's disease. J. Biol. Chem. 2003;278:46432–46439. - PubMed
-
- Youdim MBH, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neuroscience. 2006;7:295–309. - PubMed
-
- Li M, Hubalek F, Newton-Vinson P, Edmondson DE. High-level expression of human liver monoamine oxidase A in Pichia pastoris: comparison with the enzyme expressed in Saccharomyces cerevisiae. Protein Expr. Purif. 2002;24:152–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

