Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription
- PMID: 20079801
- PMCID: PMC2829939
- DOI: 10.1016/j.exphem.2010.01.002
Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription
Abstract
Objective: Microvesicles have been shown to mediate intercellular communication. Previously, we have correlated entry of murine lung-derived microvesicles into murine bone marrow cells with expression of pulmonary epithelial cell-specific messenger RNA (mRNA) in these marrow cells. The present studies establish that entry of lung-derived microvesicles into marrow cells is a prerequisite for marrow expression of pulmonary epithelial cell-derived mRNA.
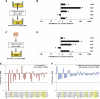
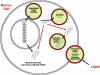
Materials and methods: Murine bone marrow cells cocultured with rat lung, but separated from them using a cell-impermeable membrane (0.4-microm pore size), were analyzed using species-specific primers (for rat or mouse).
Results: These studies revealed that surfactant B and C mRNA produced by murine marrow cells were of both rat and mouse origin. Similar results were obtained using murine lung cocultured with rat bone marrow cells or when bone marrow cells were analyzed for the presence of species-specific albumin mRNA after coculture with rat or murine liver. These studies show that microvesicles both deliver mRNA to marrow cells and mediate marrow cell transcription of tissue-specific mRNA. The latter likely underlies the longer-term stable change in genetic phenotype that has been observed. We have also observed microRNA in lung-derived microvesicles, and studies with RNase-treated microvesicles indicate that microRNA negatively modulates pulmonary epithelial cell-specific mRNA levels in cocultured marrow cells. In addition, we have also observed tissue-specific expression of brain, heart, and liver mRNA in cocultured marrow cells, suggesting that microvesicle-mediated cellular phenotype change is a universal phenomena.
Conclusion: These studies suggest that cellular systems are more phenotypically labile than previously considered.
Copyright 2010 ISEH - Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Keller S, Sanderson MP, Stoeck A. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. - PubMed
-
- Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–78. - PubMed
-
- Morel O, Toti F, Hugel B, et al. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. - PubMed
-
- Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. - PubMed
-
- Nomura S, Nakamura T, Cone J, et al. Cytometric analysis of high shear-induced platelet microparticles and effect of cytokines on microparticle generation. Cytometry. 2000;40:173–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

