Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1)
- PMID: 20079836
- PMCID: PMC2862847
- DOI: 10.1016/j.matbio.2010.01.002
Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1)
Abstract
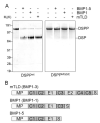
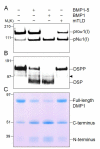
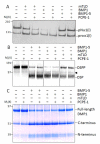
The protease that cleaves the most abundant non-collagenous protein of dentin matrix, dentin sialophosphoprotein (DSPP), into its two final dentin matrix products, dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), has not been directly identified. In this study, full-length recombinant mouse DSPP was made for the first time in furin-deficient mammalian LoVo cells and used to test the ability of three different isoforms of one candidate protease, bone morphogenetic protein-1 (BMP1) to cleave DSPP at the appropriate site. Furthermore, two reported enhancers of BMP1/mTLD activity (procollagen C-endopeptidase enhancer-1, PCPE-1, and secreted frizzled-related protein-2, sFRP2) were tested for their abilities to modulate BMP1-mediated processing of both DSPP and another SIBLING family member with a similar cleavage motif, dentin matrix protein-1 (DMP1). Three splice variants of BMP1 (classic BMP1, the full-length mTolloid (mTLD), and the shorter isoform lacking the CUB3 domain, BMP1-5) were all shown to cleave the recombinant DSPP in vitro although mTLD was relatively inefficient at processing both DSPP and DMP1. Mutation of the MQGDD peptide motif to IEGDD completely eliminated the ability of all three recombinant isoforms to process full-length recombinant DSPP in vitro thereby verifying the single predicted cleavage site. Furthermore when human bone marrow stromal cells (which naturally express furin-activated BMP1) were transduced with the adenovirus-encoding either wild-type or mutant DSPP, they were observed to fully cleave wild-type DSPP but failed to process the mutant DSPP(MQDeltaIE) during biogenesis. All three BMP1 isoforms were shown to process type I procollagen as well as DSPP and DMP1 much more efficiently in low-salt buffer (< or = 50 mM NaCl) compared to commonly used normal saline buffers (150 mM NaCl). Neither PCPE-1 nor sFRP2 were able to enhance any of the three BMP1 isoforms in cleaving either DSPP or DMP1 under either low or normal saline conditions. Interestingly, we were unable to reproduce sFRP2's reported ability to enhance the processing of type I procollagen by BMP1/mTLD. In summary, three isoforms of BMP1 process both DSPP and DMP1 at the MQX/DDP motif, but the identity of a protein that can enhance the cleavage of the two SIBLING proteins remains elusive.
Published by Elsevier B.V.
Figures



Similar articles
-
Partial blocking of mouse DSPP processing by substitution of Gly(451)-Asp(452) bond suggests the presence of secondary cleavage site(s).Connect Tissue Res. 2012;53(4):307-12. doi: 10.3109/03008207.2011.650301. Epub 2011 Dec 16. Connect Tissue Res. 2012. PMID: 22175728 Free PMC article.
-
Site specificity of DSP-PP cleavage by BMP1.Connect Tissue Res. 2014 Aug;55 Suppl 1(0 1):142-5. doi: 10.3109/03008207.2014.923863. Connect Tissue Res. 2014. PMID: 25158199 Free PMC article.
-
Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp).J Bone Miner Res. 2011 Jan;26(1):220-8. doi: 10.1002/jbmr.202. J Bone Miner Res. 2011. PMID: 20687161 Free PMC article.
-
Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis.Crit Rev Oral Biol Med. 2004 Jun 4;15(3):126-36. doi: 10.1177/154411130401500302. Crit Rev Oral Biol Med. 2004. PMID: 15187031 Review.
-
The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein.Int J Oral Sci. 2018 Nov 5;10(4):31. doi: 10.1038/s41368-018-0035-9. Int J Oral Sci. 2018. PMID: 30393383 Free PMC article. Review.
Cited by
-
Dynamics of the secreted frizzled related protein Sizzled and potential implications for binding to bone morphogenetic protein-1 (BMP-1).Sci Rep. 2022 Sep 1;12(1):14850. doi: 10.1038/s41598-022-18795-4. Sci Rep. 2022. PMID: 36050373 Free PMC article.
-
Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis.J Biol Chem. 2012 Aug 31;287(36):30426-35. doi: 10.1074/jbc.M112.388587. Epub 2012 Jul 13. J Biol Chem. 2012. PMID: 22798071 Free PMC article.
-
Metalloproteinases in Drosophila to humans that are central players in developmental processes.J Biol Chem. 2011 Dec 9;286(49):41905-41911. doi: 10.1074/jbc.R111.299768. Epub 2011 Oct 25. J Biol Chem. 2011. PMID: 22027825 Free PMC article. Review.
-
Dentin: structure, composition and mineralization.Front Biosci (Elite Ed). 2011 Jan 1;3(2):711-35. doi: 10.2741/e281. Front Biosci (Elite Ed). 2011. PMID: 21196346 Free PMC article. Review.
-
Research progress of biomimetic materials in oral medicine.J Biol Eng. 2023 Nov 23;17(1):72. doi: 10.1186/s13036-023-00382-4. J Biol Eng. 2023. PMID: 37996886 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

