The kinetic and chemical mechanism of high-fidelity DNA polymerases
- PMID: 20079883
- PMCID: PMC3047511
- DOI: 10.1016/j.bbapap.2010.01.006
The kinetic and chemical mechanism of high-fidelity DNA polymerases
Abstract
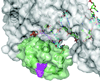
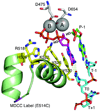
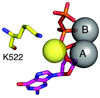
This review summarizes our current understanding of the structural, kinetic and thermodynamic basis for the extraordinary accuracy of high-fidelity DNA polymerases. High-fidelity DNA polymerases, such as the enzyme responsible for the replication of bacteriophage T7 DNA, discriminate against similar substrates with an accuracy that approaches one error in a million base pairs while copying DNA at a rate of approximately 300 base pairs per second. When the polymerase does make an error, it stalls, giving time for the slower proofreading exonuclease to remove the mismatch so that the overall error frequency approaches one in a billion. Structural analysis reveals a large change in conformation after nucleotide binding from an open to a closed state. Kinetic analysis has shown that the substrate-induced structural change plays a key role in the discrimination between correct and incorrect base pairs by governing whether a nucleotide will be retained and incorporated or rapidly released.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures
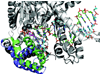

References
-
- Doublie S, Tabor S, Long AM, Richardson CC, Ellenberger T. Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 A resolution. Nature. 1998;391:251–258. - PubMed
-
- Huang HF, Chopra R, Verdine GL, Harrison SC. Structure of a covalently trapped catalytic complex of HIV-I reverse transcriptase: Implications for drug resistance. Science. 1998;282:1669–1675. - PubMed
-
- Sarafianos SG, Clark AD, Jr, Tuske S, Squire CJ, Das K, Sheng D, Ilankumaran P, Ramesha AR, Kroth H, Sayer JM, Jerina DM, Boyer PL, Hughes SH, Arnold E. Trapping HIV-1 reverse transcriptase before and after translocation on DNA. J.Biol.Chem. 2003;278:16280–16288. - PubMed
-
- Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of Ternary Complexes of Rat Dna-Polymerase-Beta, A Dna Template-Primer, and Ddctp. Science. 1994;264:1891–1903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

