Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice
- PMID: 20079918
- PMCID: PMC2830294
- DOI: 10.1016/j.virol.2009.12.011
Exacerbation of corneal scarring in HSV-1 gK-immunized mice correlates with elevation of CD8+CD25+ T cells in corneas of ocularly infected mice
Abstract
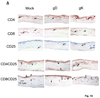
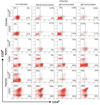
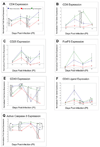
We have shown previously that exacerbation of corneal scarring (CS) in HSV-1 glycoprotein K (gK) immunized mice was associated with CD8+ T cells. In this study, we investigated the type and the nature of the immune responses that are involved in the exacerbation of CS in gK-immunized animals. BALB/c mice were vaccinated with baculovirus expressed gK, gD, or mock-immunized. Twenty-one days after the third immunization, mice were ocularly infected with 2 x 10(5) PFU/eye of virulent HSV-1 strain McKrae. Infiltration of the cornea by CD4+, CD8+, CD25+, CD4+CD25+, CD8+CD25+, CD19+, CD40+, CD40L+, CD62L+, CD95+, B7-1+, B7-2+, MHC-I+, and MHC-II+ cells was monitored by immunohistochemistry, qRT-PCR and FACS at various times post-infection (PI). This study demonstrated for the first time that the presence of CD8+CD25+ T cells in the cornea is correlated with exacerbation of CS in the gK-immunized group.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures





References
-
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1(4):247–260. - PubMed
-
- Bagenstose LM, Agarwal RK, Silver PB, Harlan DM, Hoffmann SC, Kampen RL, Chan CC, Caspi RR. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol. 2005;175(1):124–130. - PubMed
-
- Banerjee K, Deshpande S, Zheng M, Kumaraguru U, Schoenberger SP, Rouse BT. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol. 2002;219(2):108–118. - PubMed
-
- Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Wilhelmus KR, Kaufman HE, Sugar J, Hyndiuk RA, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1871–1882. - PubMed
-
- Brandt CR. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp Eye Res. 2005;80(5):607–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

