Comparative analysis of membrane-associated fusion peptide secondary structure and lipid mixing function of HIV gp41 constructs that model the early pre-hairpin intermediate and final hairpin conformations
- PMID: 20080102
- PMCID: PMC2830311
- DOI: 10.1016/j.jmb.2010.01.018
Comparative analysis of membrane-associated fusion peptide secondary structure and lipid mixing function of HIV gp41 constructs that model the early pre-hairpin intermediate and final hairpin conformations
Abstract
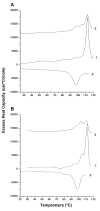
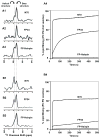
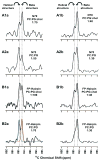
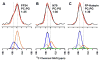
Fusion between viral and host cell membranes is the initial step of human immunodeficiency virus infection and is mediated by the gp41 protein, which is embedded in the viral membrane. The approximately 20-residue N-terminal fusion peptide (FP) region of gp41 binds to the host cell membrane and plays a critical role in fusion catalysis. Key gp41 fusion conformations include an early pre-hairpin intermediate (PHI) characterized by extended coiled-coil structure in the region C-terminal of the FP and a final hairpin state with compact six-helix bundle structure. The large "N70" (gp41 1-70) and "FP-Hairpin" constructs of the present study contained the FP and respectively modeled the PHI and hairpin conformations. Comparison was also made to the shorter "FP34" (gp41 1-34) fragment. Studies were done in membranes with physiologically relevant cholesterol content and in membranes without cholesterol. In either membrane type, there were large differences in fusion function among the constructs with little fusion induced by FP-Hairpin, moderate fusion for FP34, and very rapid fusion for N70. Overall, our findings support acceleration of gp41-induced membrane fusion by early PHI conformation and fusion arrest after folding to the final six-helix bundle structure. FP secondary structure at Leu7 of the membrane-associated constructs was probed by solid-state nuclear magnetic resonance and showed populations of molecules with either beta-sheet or helical structure with greater beta-sheet population observed for FP34 than for N70 or FP-Hairpin. The large differences in fusion function among the constructs were not obviously correlated with FP secondary structure. Observation of cholesterol-dependent FP structure for fusogenic FP34 and N70 and cholesterol-independent structure for non-fusogenic FP-Hairpin was consistent with membrane insertion of the FP for FP34 and N70 and with lack of insertion for FP-Hairpin. Membrane insertion of the FP may therefore be associated with the early PHI conformation and FP withdrawal with the final hairpin conformation.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures
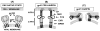

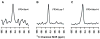
Similar articles
-
Biochemistry and biophysics of HIV-1 gp41 - membrane interactions and implications for HIV-1 envelope protein mediated viral-cell fusion and fusion inhibitor design.Curr Top Med Chem. 2011 Dec;11(24):2959-84. doi: 10.2174/156802611798808497. Curr Top Med Chem. 2011. PMID: 22044229 Free PMC article. Review.
-
Solid-state NMR spectroscopy of the HIV gp41 membrane fusion protein supports intermolecular antiparallel β sheet fusion peptide structure in the final six-helix bundle state.J Mol Biol. 2014 Mar 6;426(5):1077-94. doi: 10.1016/j.jmb.2013.11.010. Epub 2013 Nov 16. J Mol Biol. 2014. PMID: 24246500 Free PMC article.
-
HIV gp41 six-helix bundle constructs induce rapid vesicle fusion at pH 3.5 and little fusion at pH 7.0: understanding pH dependence of protein aggregation, membrane binding, and electrostatics, and implications for HIV-host cell fusion.Eur Biophys J. 2011 Apr;40(4):489-502. doi: 10.1007/s00249-010-0662-3. Epub 2011 Jan 11. Eur Biophys J. 2011. PMID: 21222118 Free PMC article.
-
Solid-state nuclear magnetic resonance measurements of HIV fusion peptide 13CO to lipid 31P proximities support similar partially inserted membrane locations of the α helical and β sheet peptide structures.J Phys Chem A. 2013 Oct 3;117(39):9848-59. doi: 10.1021/jp312845w. Epub 2013 Feb 28. J Phys Chem A. 2013. PMID: 23418890 Free PMC article.
-
Peptide and non-peptide HIV fusion inhibitors.Curr Pharm Des. 2002;8(8):563-80. doi: 10.2174/1381612024607180. Curr Pharm Des. 2002. PMID: 11945159 Review.
Cited by
-
The three lives of viral fusion peptides.Chem Phys Lipids. 2014 Jul;181:40-55. doi: 10.1016/j.chemphyslip.2014.03.003. Epub 2014 Apr 2. Chem Phys Lipids. 2014. PMID: 24704587 Free PMC article. Review.
-
Lipid acyl chain protrusion induced by the influenza virus hemagglutinin fusion peptide detected by NMR paramagnetic relaxation enhancement.Biophys Chem. 2023 Aug;299:107028. doi: 10.1016/j.bpc.2023.107028. Epub 2023 May 13. Biophys Chem. 2023. PMID: 37247572 Free PMC article.
-
Biochemistry and biophysics of HIV-1 gp41 - membrane interactions and implications for HIV-1 envelope protein mediated viral-cell fusion and fusion inhibitor design.Curr Top Med Chem. 2011 Dec;11(24):2959-84. doi: 10.2174/156802611798808497. Curr Top Med Chem. 2011. PMID: 22044229 Free PMC article. Review.
-
Antimicrobial and cell-penetrating peptides induce lipid vesicle fusion by folding and aggregation.Eur Biophys J. 2012 Feb;41(2):177-87. doi: 10.1007/s00249-011-0771-7. Epub 2011 Nov 12. Eur Biophys J. 2012. PMID: 22080286 Free PMC article.
-
Hydrogen-Deuterium Exchange Supports Independent Membrane-Interfacial Fusion Peptide and Transmembrane Domains in Subunit 2 of Influenza Virus Hemagglutinin Protein, a Structured and Aqueous-Protected Connection between the Fusion Peptide and Soluble Ectodomain, and the Importance of Membrane Apposition by the Trimer-of-Hairpins Structure.Biochemistry. 2019 May 14;58(19):2432-2446. doi: 10.1021/acs.biochem.8b01272. Epub 2019 May 1. Biochemistry. 2019. PMID: 31008587 Free PMC article.
References
-
- Furuta RA, Wild CT, Weng Y, Weiss CD. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. - PubMed
-
- Bewley CA, Louis JM, Ghirlando R, Clore GM. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J Biol Chem. 2002;277:14238–14245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

