Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina
- PMID: 20080108
- PMCID: PMC2828864
- DOI: 10.1016/j.mvr.2010.01.006
Chronic whole-body hypoxia induces intussusceptive angiogenesis and microvascular remodeling in the mouse retina
Abstract
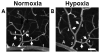
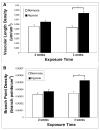
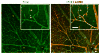
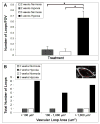
Currently, little is known about the response of the adult retinal microvasculature to hypoxia. To test the hypothesis that chronic systemic hypoxia induces angiogenesis and microvascular remodeling in the adult mouse retina, adult 10-week old female C57Bl/6 mice were exposed to 10% O(2) for 2 or 3 weeks. After hypoxia exposure, retinas were harvested, whole-mounted, and processed for immunohistochemistry. Retinas were stained with lectin, anti-smooth muscle alpha-actin antibody, and anti-NG2 antibody to visualize microvascular networks and their cellular components. Confocal microscopy was used to obtain images of superficial retinal networks. Images were analyzed to assess vessel diameter, vascular length density, branch point density, and the presence of vascular loops, a hallmark of intussusceptive angiogenesis. Both 2 and 3 weeks of hypoxia exposure resulted in a significant increase in the diameters of arterioles and post-arteriole capillaries (p<0.003). After 3 weeks of hypoxia, vascular length density and branch point density were significantly increased in retinas exposed to hypoxia as compared to normoxic controls (p<0.001). The number of vascular loops in the superficial retinal networks was significantly greater in hypoxia-exposed retinas (p < or = 0.001). Our results demonstrate, for the first time, intussusceptive angiogenesis as a tissue-level mechanism of vascular adaptation to chronic systemic hypoxia in the adult mouse retina and contribute to our understanding of hypoxia-induced angiogenesis and microvascular remodeling in the adult animal.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Retinal hypoxia and angiogenesis with methamphetamine.Exp Eye Res. 2021 May;206:108540. doi: 10.1016/j.exer.2021.108540. Epub 2021 Mar 15. Exp Eye Res. 2021. PMID: 33736986 Free PMC article.
-
Quantification of vascular tortuosity as an early outcome measure in oxygen induced retinopathy (OIR).Exp Eye Res. 2014 Mar;120:55-60. doi: 10.1016/j.exer.2013.12.020. Epub 2014 Jan 10. Exp Eye Res. 2014. PMID: 24418725
-
Retinal oxygen distribution. Its role in the physiopathology of vasoproliferative microangiopathies.Retina. 1995;15(4):332-47. Retina. 1995. PMID: 8545580 Review.
-
Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity.Invest Ophthalmol Vis Sci. 2003 Jul;44(7):3119-23. doi: 10.1167/iovs.02-1122. Invest Ophthalmol Vis Sci. 2003. PMID: 12824260
-
Intussusceptive angiogenesis: expansion and remodeling of microvascular networks.Angiogenesis. 2014 Jul;17(3):499-509. doi: 10.1007/s10456-014-9428-3. Epub 2014 Mar 26. Angiogenesis. 2014. PMID: 24668225 Free PMC article. Review.
Cited by
-
Silibinin inhibits VEGF secretion and age-related macular degeneration in a hypoxia-dependent manner through the PI-3 kinase/Akt/mTOR pathway.Br J Pharmacol. 2013 Feb;168(4):920-31. doi: 10.1111/j.1476-5381.2012.02227.x. Br J Pharmacol. 2013. PMID: 23004355 Free PMC article.
-
Single-Cell Transcriptome Analysis Reveals Embryonic Endothelial Heterogeneity at Spatiotemporal Level and Multifunctions of MicroRNA-126 in Mice.Arterioscler Thromb Vasc Biol. 2022 Mar;42(3):326-342. doi: 10.1161/ATVBAHA.121.317093. Epub 2022 Jan 13. Arterioscler Thromb Vasc Biol. 2022. PMID: 35021856 Free PMC article.
-
Epicatechin Gallate Protects HBMVECs from Ischemia/Reperfusion Injury through Ameliorating Apoptosis and Autophagy and Promoting Neovascularization.Oxid Med Cell Longev. 2019 Mar 6;2019:7824684. doi: 10.1155/2019/7824684. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30962864 Free PMC article.
-
Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection.Elife. 2018 Mar 21;7:e34861. doi: 10.7554/eLife.34861. Elife. 2018. PMID: 29561727 Free PMC article.
-
BMP-SMAD1/5 Signaling Regulates Retinal Vascular Development.Biomolecules. 2020 Mar 23;10(3):488. doi: 10.3390/biom10030488. Biomolecules. 2020. PMID: 32210087 Free PMC article.
References
-
- Antonetti DA, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11. - PubMed
-
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473–83. - PubMed
-
- Burri PH, Djonov V. Intussusceptive angiogenesis--the alternative to capillary sprouting. Mol Aspects Med. 2002;23:S1–27. - PubMed
-
- Burri PH, et al. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

