Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells
- PMID: 20080162
- PMCID: PMC2849731
- DOI: 10.1016/j.ijpharm.2010.01.019
Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells
Abstract
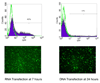
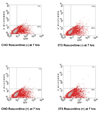
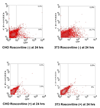
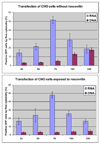
The design of appropriate gene delivery systems is essential for the successful application of gene therapy to clinical medicine. Cationic lipid-mediated delivery is a viable alternative to viral vector-mediated gene delivery in applications where transient gene expression is desirable. However, cationic lipid-mediated delivery of DNA to post-mitotic cells such as neurons is often reported to be of low efficiency, due to the presumed inability of the DNA to translocate to the nucleus. Lipid-mediated delivery of RNA is an attractive alternative to non-viral DNA delivery in some clinical applications, because transit across the nuclear membrane is not necessary. Here we report a comparative investigation of cationic lipid-mediated delivery of RNA versus DNA vectors encoding the reporter gene green fluorescent protein (GFP) in Chinese Hamster Ovary (CHO) and NIH3T3 cells following chemical inhibition of proliferation, and in primary mixed neuronal cell cultures. Using optimized formulations and transfection procedures, we assess gene expression by flow cytometry to specifically address some of the advantages and disadvantages of lipid-mediated RNA and DNA gene transfer. Despite inhibition of cell proliferation, over 45% of CHO cells express GFP after lipid-mediated transfection with RNA vectors. Transfection efficiency of DNA encoding GFP in proliferation-inhibited CHO cells was less than 5%. Detectable expression after RNA transfection occurs at least 3h earlier than after DNA transfection, but DNA transfection eventually produces a mean level of per cell GFP expression (as assayed by flow cytometry) that is higher than after RNA transfection. Transfection of proliferation-inhibited NIH3T3 cells and primary mixed neuronal cultures produced similar results, with RNA encoded GFP expression in 2-4 times the number of cells as after DNA encoded GFP expression. These results demonstrate the increased efficiency of RNA transfection relative to DNA transfection in non-dividing cells. We used firefly luciferase encoded by RNA and DNA vectors to investigate the time course of gene expression after delivery of RNA or DNA to primary neuronal cortical cells. Delivery of mRNA resulted in rapid onset (within 1h) of luciferase expression after transfection, a peak in expression 5-7h after transfection, and a return to baseline within 12h after transfection. After DNA delivery significant luciferase activity did not appear until 7h after transfection, but peak luciferase expression was always at least one order of magnitude higher than after RNA delivery. The peak expression after luciferase-expressing DNA delivery occurred 36-48 h after transfection and remained at a significant level for at least one week before dropping to baseline. This observation is consistent with our in vivo delivery results, which are shown as well. RNA delivery may therefore be more suitable for short-term transient gene expression due to rapid onset, shorter duration of expression and greater efficiency, particularly in non-dividing cells. Higher mean levels of expression per cell obtained following DNA delivery and the longer duration of expression confirm a continuing role for DNA gene delivery in clinical applications that require longer term transient gene expression.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Journal of Gene Medicine. 2008. Web site. http://www.wiley.co.uk/genetherapy/
-
- Anderson DM, Hall LL, Ayyalapu A, Irion VR, Nantz MH, Hecker JG. Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for non-viral mRNA gene delivery to the CNS. Human Gene Therapy. 2003;14:191–202. - PubMed
-
- Aronsohn AI, Hughes JA. Nuclear localization signal peptides enhance cationic liposome-mediated gene therapy. Journal of Drug Targeting. 1998;5:163–169. - PubMed
-
- Balasubramaniam RP, Bennett MJ, Aberle AM, Malone JG, Nantz MH, Malone RW. Structural and functional analysis of cationic transfection lipids: The hydrophobic domain. Gene Therapy. 1996;3:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

