The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering
- PMID: 20080298
- PMCID: PMC2826798
- DOI: 10.1016/j.biomaterials.2009.12.053
The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering
Abstract
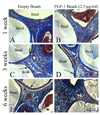
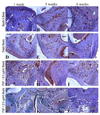
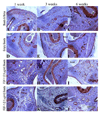
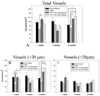
Engineered vascularized adipose tissue could serve as an alternative to traditional tissue reconstruction procedures. Adipose formation occurs in a coordinated fashion with neovascularization. Previous studies have shown that extracellular matrix-based materials supplemented with factors that stimulate neovascularization promote adipogenesis in a number of animal models. The present study examines the ability of fibroblast growth factor (FGF-1) delivered from alginate microbeads to induce neovascularization and adipogenesis in type I collagen gels in a vascular pedicle model of adipose tissue engineering. FGF-1 loaded microbeads stimulated greater vascular network formation in an in vitro 3D co-culture model than a single bolus of FGF-1. In in vivo studies, FGF-1 loaded beads suspended in collagen and implanted in a chamber surrounding the exposed femoral pedicle of a rat resulted in a significant increase in vascular density at 1 and 6 weeks in comparison to bolus administration of FGF-1. Staining for smooth muscle actin showed that over 48% of vessels had associated mural cells. While an increase in neovascularization was achieved, there was less than 3% adipose under any condition. These results show that delivery of FGF-1 from alginate beads stimulated a more persistent neovascularization response than bolus FGF-1 both in vitro and in vivo. However, unlike previous studies, this increased neovascularization did not result in adipogenesis. Future studies need to provide a better understanding of the relationship between neovascularization and adipogenesis in order to design advanced tissue engineering therapies.
2009 Elsevier Ltd. All rights reserved.
Figures





References
-
- Patrick CW. Breast tissue engineering. Annu Rev Biomed Eng. 2004;6:109–130. - PubMed
-
- Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354 Suppl 1:SI32–SI34. - PubMed
-
- Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. - PubMed
-
- Morrison WA. Progress in tissue engineering of soft tissue and organs. Surgery. 2009;145(2):127–130. - PubMed
-
- Uriel S, Brey EM, Greisler HP. Sustained low levels of fibroblast growth factor-1 promote persistent microvascular network formation. Am J Surg. 2006;192(5):604–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

