Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition
- PMID: 20080541
- PMCID: PMC2824382
- DOI: 10.1073/pnas.0908953107
Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition
Abstract
An additional copy of the beta-amyloid precursor protein (APP) gene causes early-onset Alzheimer's disease (AD) in trisomy 21 (DS). Endosome dysfunction develops very early in DS and AD and has been implicated in the mechanism of neurodegeneration. Here, we show that morphological and functional endocytic abnormalities in fibroblasts from individuals with DS are reversed by lowering the expression of APP or beta-APP-cleaving enzyme 1 (BACE-1) using short hairpin RNA constructs. By contrast, endosomal pathology can be induced in normal disomic (2N) fibroblasts by overexpressing APP or the C-terminal APP fragment generated by BACE-1 (betaCTF), all of which elevate the levels of betaCTFs. Expression of a mutant form of APP that cannot undergo beta-cleavage had no effect on endosomes. Pharmacological inhibition of APP gamma-secretase, which markedly reduced Abeta production but raised betaCTF levels, also induced AD-like endosome dysfunction in 2N fibroblasts and worsened this pathology in DS fibroblasts. These findings strongly implicate APP and the betaCTF of APP, and exclude Abeta and the alphaCTF, as the cause of endocytic pathway dysfunction in DS and AD, underscoring the potential multifaceted value of BACE-1 inhibition in AD therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
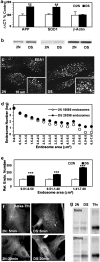
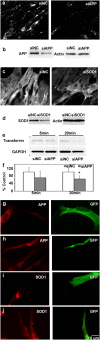

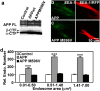
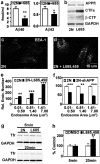

Comment in
-
Excess betaCTF, not Abeta: the culprit in Alzheimer-related endocytic dysfunction.Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1263-4. doi: 10.1073/pnas.0913922107. Proc Natl Acad Sci U S A. 2010. PMID: 20133888 Free PMC article. No abstract available.
References
-
- Haass C, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. - PubMed
-
- Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992;255:728–730. - PubMed
-
- Sisodia SS, Price DL. Role of the beta-amyloid protein in Alzheimer’s disease. FASEB J. 1995;9:366–370. - PubMed
-
- Athan ES, Lee JH, Arriaga A, Mayeux RP, Tycko B. Polymorphisms in the promoter of the human APP gene: Functional evaluation and allele frequencies in Alzheimer disease. Arch Neurol. 2002;59:1793–1799. - PubMed
-
- Rovelet-Lecrux A, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

